 | |
| Names | |
|---|---|
| Other names Yttrium triperchlorate, yttrium(III) perchlorate | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| ChemSpider | |
| ECHA InfoCard | 100.034.388 |
| EC Number |
|
| PubChem CID | |
InChI
| |
SMILES
| |
| Properties | |
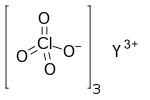
| Chemical formula | Y(ClO 4) 3 |
| Molar mass | 387.244 |
| Appearance | liquid |
| Density | g/cm |
| Solubility in water | soluble |
| Hazards | |
| Occupational safety and health (OHS/OSH): | |
| Main hazards | Oxidizer |
| GHS labelling: | |
| Pictograms |  
|
| Signal word | Danger |
| Precautionary statements | P210, P220, P260, P264, P280, P301+P330+P331, P302+P361+P354, P304+P340, P305+P354+P338, P316, P321, P363, P370+P378, P405, P501 |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa). Infobox references | |
Yttrium perchlorate is the inorganic compound with the chemical formula Y(ClO
4)
3. The compound is an yttrium salt of perchloric acid.
Synthesis
Dissolving yttrium oxide in perchloric acid solution can produce yttrium perchlorate octahydrate.
Chemical properties
Potentially explosive.
Physical properties
The compound is soluble in water and forms a hexahydrate with the formula Y(ClO
4)
3•6H
2O.
References
- "Yttrium(III) Perchlorate Solution". American Elements. Retrieved 14 March 2023.
- "CAS 14017-56-2 Yttrium perchlorate - Alfa Chemistry". alfa-chemistry.com. Retrieved 14 March 2023.
- "Yttrium(III) perchlorate, 50% w/w aq. soln., Reagent Grade, Thermo Scientific". Fisher Scientific. Retrieved 14 March 2023.
- Macintyre, Jane E. (13 November 1994). Dictionary of Inorganic Compounds, Supplement 2. CRC Press. p. 585. ISBN 978-0-412-49100-9. Retrieved 15 March 2023.
- Macintyre, Jane E. (23 July 1992). Dictionary of Inorganic Compounds. CRC Press. p. 2931. ISBN 978-0-412-30120-9. Retrieved 15 March 2023.
- "Yttrium Perchlorate, Hydrated, 50% Solution, Reagent". gfschemicals.com. Retrieved 14 March 2023.
- "40580 Yttrium(III) perchlorate, 50% w/w aq. soln., Reagent Grade". Alfa Aesar. Retrieved 14 March 2023.
| Yttrium compounds | |||
|---|---|---|---|
| Yttrium(II) | |||
| Yttrium(III) |
| ||