 | |
| Names | |
|---|---|
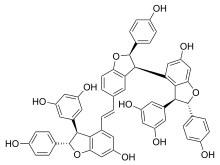
| Preferred IUPAC name (2S,2S,3R,3S,4E,6S,6S)-2,3,6-Tris(4-hydroxyphenyl)-2,2,3,3,6,6-hexahydro-2(3,4),3(3,5),6(4,3)-tris(benzofurana)-1,7(1)-dibenzenaheptaphan-4-ene-1,1,2,6,7,7-hexol | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| ChEMBL | |
| ChemSpider | |
| PubChem CID | |
| UNII | |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | C56H42O12 |
| Molar mass | 906.940 g·mol |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |
Vitisin C is a hydroxystilbenoid. It is a resveratrol tetramer found in plants of the genus Vitis (grapevines).
References
- Ito, J (1996). "Absolute structures of new hydroxystilbenoids, vitisin C and viniferal, from Vitis vinifera 'Kyohou'". Tetrahedron. 52 (30): 9991–9998. doi:10.1016/0040-4020(96)00543-1.
- Seya, K; Furukawa, K; Taniguchi, S; Kodzuka, G; Oshima, Y; Niwa, M; Motomura, S (2003). "Endothelium-dependent vasodilatory effect of vitisin C, a novel plant oligostilbene from Vitis plants (Vitaceae), in rabbit aorta". Clinical Science. 105 (1): 73–9. doi:10.1042/CS20020288. PMID 12605596. S2CID 41885739.
External links
| Oligostilbenoids and their glycosides | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dimers |
| ||||||||||||
| Trimers | |||||||||||||
| Tetramers: |
| ||||||||||||
| Higher polymers (five units or more) |
| ||||||||||||
| Oligomeric forms of resveratrol |
| ||||||||||||
| Glycosides or conjugates |
| ||||||||||||
This article about an aromatic compound is a stub. You can help Misplaced Pages by expanding it. |