 | |
| Names | |
|---|---|
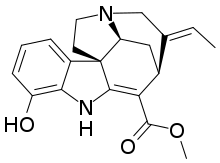
| IUPAC name Methyl (19E)-12-hydroxy-2,16-didehydrocur-19-en-17-oate | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| ChEBI | |
| ChemSpider | |
| PubChem CID | |
| UNII | |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | C24H39NO7 |
| Molar mass | 453.576 g·mol |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa). Infobox references | |
Vinervine is a monoterpene indole alkaloid of the Vinca sub-group. It is a derivative of akuammicine, with one additional hydroxy (OH) group in the indole portion, hence it is also known as 12-hydroxyakuammicine.
History
The alkaloids are a large group of natural products which are classified according to the part-structure which members of a particular group contain. Vinervine is a monoterpene indole alkaloid of the Vinca sub-group which shares a common biosynthesis with other members, namely that they are derived from strictosidine. It was first characterised in 1964 and the structures of closely related materials including akuammicine were confirmed in 1983.
Natural occurrence

Vinervine is found in a variety of plants of the Apocynaceae family, including Vinca erecta, Tabernaemontana divaricata and several other flowering plants species that are native to Africa, Asia, and Europe.
Biosynthesis
Main article: Indole alkaloid § BiosynthesisAs with other indole alkaloids, the biosynthesis of vinervine starts from the amino acid tryptophan. This is converted into strictosidine before further elaboration.
Research
Plant metabolites have long been studied for their biological activity and alkaloids in particular are major subjects for ethnobotanical research. However, vinervine has had little reported utility.
See also
References
- ^ Dewick, Paul M (2002). Medicinal Natural Products. A Biosynthetic Approach. Second Edition. Wiley. pp. 350–359. ISBN 0-471-49640-5.
- Saxton JE (1984). "Recent progress in the chemistry of indole alkaloids and mould metabolites". Natural Product Reports. 1: 21. doi:10.1039/NP9840100021.
- ^ Abdurakhimova N, Yuldashev PK, Yunusov, SY (1964). "Pseudokopsinine—a new alkaloid from aerial parts of Vinca erecta". Doklady Akademii Nauk SSSR (in Russian). 21 (2): 29–31.
- Yagudaev MR (1983). "NMR investigation of alkaloids. IV. C NMR spectra and structures of norfluorocurarine, akuammicine, vincanidine, and vinervinine". Chemistry of Natural Compounds. 19 (2): 199–201. doi:10.1007/BF00580558. S2CID 28255077.
- Yuldashev PK, Ubaev U, Kuchenkova MA, Yunusov SY (January 1965). "Structure of vincanidine and vinervine". Chemistry of Natural Compounds. 1 (1): 25–30. doi:10.1007/BF00571576. S2CID 27933208.
- Kuchenkova MA, Yuldashev PK, Yunusov SY (1965). "Vinervine — A new alkaloid from the aboveground part of Vinca erecta". Bulletin of the Academy of Sciences, USSR Division of Chemical Science. 14 (12): 2119–2121. doi:10.1007/BF00845999.
- Pawelka KH, Stöckigt J (April 1983). "Indole alkaloids from cell suspension cultures of Tabernaemontana divaricata and Tabernanthe iboga". Plant Cell Reports. 2 (2): 105–7. doi:10.1007/BF00270178. PMID 24257961. S2CID 23570705.
- ^ Pratchayasakul W, Pongchaidecha A, Chattipakorn N, Chattipakorn S (April 2008). "Ethnobotany & ethnopharmacology of Tabernaemontana divaricata". The Indian Journal of Medical Research. 127 (4): 317–35. PMID 18577786. S2CID 1119874.
- Babiaka SB, Ntie-Kang F, Lifongo LL, Ndingkokhar B, Mbah JA, Yong JN (2015). "The chemistry and bioactivity of Southern African flora I: A bioactivity versus ethnobotanical survey of alkaloid and terpenoid classes". RSC Advances. 5 (54): 43242–43267. Bibcode:2015RSCAd...543242B. doi:10.1039/C5RA01912E.
- Ghisalberti EL, Pennacchio M, Alexander E (1998). "Survey of Secondary Plant Metabolites with Cardiovascular Activity". Pharmaceutical Biology. 36 (4): 259. doi:10.1076/phbi.36.4.237.4583.
- Heijden R, Jacobs D, Snoeijer W, Hallard D, Verpoorte R (2004). "The Catharanthus Alkaloids:Pharmacognosy and Biotechnology". Current Medicinal Chemistry. 11 (5): 607–628. doi:10.2174/0929867043455846. PMID 15032608.
Further reading
- Edwin Saxton J (15 September 2009). Indoles, Part 4: The Monoterpenoid Indole Alkaloids. John Wiley & Sons. ISBN 978-0-470-18844-6.