 | |
 | |
| Names | |
|---|---|
| Preferred IUPAC name Thianthrene | |
| Other names Thianthren; 9,10-Dithiaanthracene; Di-o-phenylene disulfide | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.001.998 |
| EC Number |
|
| PubChem CID | |
| UNII | |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | C12H8S2 |
| Molar mass | 216.32 g·mol |
| Melting point | 151 to 155 °C (304 to 311 °F; 424 to 428 K) |
| Boiling point | 364 to 366 °C (687 to 691 °F; 637 to 639 K) |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |
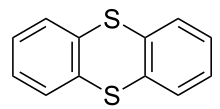
Thianthrene is a sulfur-containing heterocyclic chemical compound. It is a derivative of the parent heterocycle called dithiin. It is notable for its ease of oxidation.
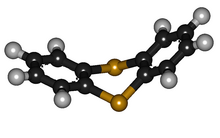
Structure and synthesis
Like other 1,4-dithiins but unlike its oxygen analog dibenzodioxin, the shape of thianthrene is not planar. It is bent, with a fold angle of 128° between the two benzo groups.
Thianthrene can be prepared by treating benzene with disulfur dichloride in the presence of aluminium chloride.
History
Thianthrene was first synthesized by John Stenhouse by dry distillation of sodium benzenesulfonate. Thianthrene is oxidized by sulfuric acid forming a red radical cation. Thianthrene has been characterized by Electron paramagnetic resonance. Four different publications describe the crystal structure of salts of thianthrene.
References
- International Union of Pure and Applied Chemistry (2014). Nomenclature of Organic Chemistry: IUPAC Recommendations and Preferred Names 2013. The Royal Society of Chemistry. p. 216. doi:10.1039/9781849733069. ISBN 978-0-85404-182-4.
- Thianthrene at Sigma-Aldrich
- Hosoya, S. (1963). "Molecular shapes of thianthrene and related heterocyclic compounds". Acta Crystallographica. 16 (4): 310–312. Bibcode:1963AcCry..16..310H. doi:10.1107/S0365110X63000797.
- Gallaher, K. L.; Bauer, S. H. (1975). "Structure and inversion potential of thianthren". Journal of the Chemical Society, Faraday Transactions 2. 71: 1173–1182. doi:10.1039/F29757101173.
- Aroney, M. J.; Le Fèvre, R. J. W.; Saxby, J. D. (1965). "92. Molecular polarisability. The apparent conformations of thianthren and of three of its oxides as solutes in benzene". Journal of the Chemical Society (Resumed): 571–575. doi:10.1039/JR9650000571.
- US patent 3997560, "Process for the manufacture of thianthrene", issued 1976-12-14 .
- Stenhouse, J. (1869). "Ueber die Producte der trockenen Destillation der sulfobenzolsauren Salze" [On the Dry Distillation Products from Sulfobenzoic Acid Salts]. Annalen der Chemie und Pharmacie (in German). 149 (2): 247–255. doi:10.1002/jlac.18691490216.
- W. Dilthey: Versammlungsberichte Bonner Chemische Gesellschaft, Angewandte Chemie, Volume 42, Issue 24, pp. 668–670, 15. June 1929; doi:10.1002/ange.19290422405.
- Shine, Henry J. (July 1998). "EPR and the History of the Thianthrene Cation Radical". Foundations of modern EPR. World Scientific. ISBN 978-981-02-3295-5.