 | |
| Names | |
|---|---|
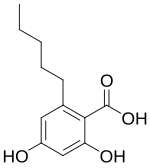
| Preferred IUPAC name 2,4-Dihydroxy-6-pentylbenzoic acid | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| PubChem CID | |
| UNII | |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | C12H16O4 |
| Molar mass | 224.256 g·mol |
| Appearance | colorless solid |
| Melting point | 148–9 °C (298–48 °F; 421–282 K) |
| Related compounds | |
| Related compounds | Cannabidiolic acid |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa). Infobox references | |
Olivetolic acid is an organic compound with the formula C5H11C6H2(OH)2CO2H. Several isomers with this formula exist. Olivetolic acid can be viewed as a derivative of olivetol ( 1,3,5-C5H11C6H2(OH)2CO2H, with a carboxylic acid group adjacent to the pentyl (C5H11) group. Olivetolic acid has attracted attention because it is an intermediate in the biosynthetic pathway of the cannabinoids, found in Cannabis sativa.
The ester dimer of olivetolic acid, anziaic acid, is found in lichen.
References
- Fellermeier, Monika; Zenk, Meinhart H (May 1998). "Prenylation of olivetolate by a hemp transferase yields cannabigerolic acid, the precursor of tetrahydrocannabinol". FEBS Letters. 427 (2): 283–285. doi:10.1016/S0014-5793(98)00450-5. PMID 9607329.
- Cheng, Bokun; Cao, Shugeng; Vasquez, Victor; Annamalai, Thirunavukkarasu; Tamayo-Castillo, Giselle; Clardy, Jon; Tse-Dinh, Yuk-Ching (8 April 2013). "Identification of Anziaic Acid, a Lichen Depside from Hypotrachyna sp., as a New Topoisomerase Poison Inhibitor". PLOS ONE. 8 (4): e60770. Bibcode:2013PLoSO...860770C. doi:10.1371/journal.pone.0060770. PMC 3620467. PMID 23593306.
- M.P. Polovinka; N.I. Komarova; D.V. Korchagina; D.N. Sokolov; O.A. Luzina; N.G. Vlasenko; A.A. Malyuga; E.V. Romanova; N.F. Salakhutdinov: Secondary metabolites of the lichen Cladonia stellaris in Chemistry of Natural Compounds 48 (2012) 392–395, doi:10.1007/s10600-012-0259-4.
This article about an organic compound is a stub. You can help Misplaced Pages by expanding it. |