| |||
| |||
| Names | |||
|---|---|---|---|
| IUPAC name Tetracyclododecane | |||
| Other names Wurtzitane | |||
| Identifiers | |||
| CAS Number | |||
| 3D model (JSmol) | |||
| ChemSpider | |||
| PubChem CID | |||
| CompTox Dashboard (EPA) | |||
InChI
| |||
SMILES
| |||
| Properties | |||
| Chemical formula | C12H18 | ||
| Molar mass | 162.276 g·mol | ||
| Melting point | 325 °C (617 °F; 598 K) | ||
| Boiling point | Sublimes | ||
| Structure | |||
| Point group | D3h | ||
| Dipole moment | 0 D | ||
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |||
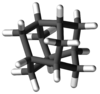
Iceane is a saturated polycyclic hydrocarbon with formula C12H18. It has a cage-like molecular structure, whose carbon skeleton can be viewed as three fused cyclohexane rings in the boat conformation; or as two such rings in the chair conformation, connected by three parallel axial bonds. The spatial arrangement of carbon atoms in iceane is the lonsdalite crystalline structure.
The name "iceane" was proposed by the chemist Louis Fieser about a decade before the compound was first prepared. He was carrying out studies on the arrangement of water molecules in ice, when it occurred to him that there could exist a stable hydrocarbon with the above structure.
It is also referred to as wurtzitane, due to its similarity to the wurtzite crystal structure; however, the name "iceane" has precedence.
See also
References
- ^ Hamon, D. P. G.; Taylor, G. F. (1976). "A synthesis of tetracyclododecane (iceane)". Aust. J. Chem. 29 (8): 1721–1734. doi:10.1071/CH9761721.
- Cupas, Chris A.; Hodakowski, Leonard (July 1974). "Iceane". Journal of the American Chemical Society. 96 (14): 4668–4669. doi:10.1021/ja00821a050.
- Fieser, L. F. (1965). "Extensions in the use of plastic tetrahedral models". J. Chem. Educ. 42 (8): 408–412. doi:10.1021/ed042p408.
- Hargittai, M.; Hargittai, I. (2013). "Polyhedral Molecular Geometries". In Senechal, M. (ed.). Shaping Space: Exploring Polyhedra in Nature, Art, and the Geometrical Imagination. Springer Science & Business Media. pp. 153–170, 299. ISBN 9780387927145.
- Tobler, H.; Klaus, R. O.; Ganter, C. (1975). "Wurtzitan (Tetracyclododecan)". Helv. Chim. Acta. 58 (5): 1455–1464. doi:10.1002/hlca.19750580522.
External links
- Royal Society of Chemistry Journal
- Symmetry Through the Eyes of a Chemist, Magdolna Hargittai
- A Basis for Synthesis Design, Tse-Lok Ho
- Structures and Energies of Polycyclic Hydrocarbons, Joan E. Shields