Chemical data page
This page provides supplementary chemical data on ethylene glycol .
Material Safety Data Sheet
The handling of this chemical may incur notable safety precautions. It is highly recommended that you seek the Material Safety Datasheet (MSDS ) for this chemical from a reliable source and follow its directions.
Structure and properties
Thermodynamic properties
Phase behavior
Triple point
256 K (−17 °C), ? Pa
Critical point
720 K (447 °C), 8.2 MPa
Standard enthalpy change of fusion , Δfus H
9.9 kJ/mol
Standard entropy change of fusion , Δfus S
38.2 J/(mol·K)
Standard enthalpy change of vaporization , Δvap H
65.6 kJ/mol
Standard entropy change of vaporization , Δvap S
? J/(mol·K)
Solid properties
Standard enthalpy change of formation , Δf H solid
? kJ/mol
Standard molar entropy , S solid
? J/(mol K)
Heat capacity , cp
? J/(mol K)
Liquid properties
Standard enthalpy change of formation , Δf H liquid
−460 kJ/mol
Standard molar entropy , S liquid
166.9 J/(mol·K)
Heat capacity , cp
149.5 J/(mol·K)
Gas properties
Standard enthalpy change of formation , Δf H gas
−3955.4 kJ/mol
Standard molar entropy , S gas
311.8 J/(mol·K)
Heat capacity , cp
78 J/(mol·K) at 25 °C
Vapor pressure of liquid
P in mm Hg 1
10
40
100
400
760
T in °C 53.0
92.1
120.0
141.8
178.5
197.3
Table data obtained from CRC Handbook of Chemistry and Physics, 44th ed.
Temperature dependence of ethylene glycol vapor pressure. Uses formula
log
e
P
[
kPa
]
=
−
25.99771
log
e
T
[
K
]
−
14768.57
T
[
K
]
+
191.4250
+
2.062331
×
10
−
05
(
T
[
K
]
)
2
{\displaystyle \log _{e}P=-25.99771\log _{e}T-{\frac {14768.57}{T}}+191.4250+2.062331\times 10^{-05}\,(T)^{2}}
Freezing point of aqueous solutions
% ethylene glycol by volume
5
10
15
20
25
30
35
40
45
50
Freezing point (°F)
−1.1
−2.2
−3.9
−6.7
−8.9
−12.8
−16.1
−20.6
−26.7
−33.2
Specific gravity d
1.004
1.006
1.012
1.017
1.020
1.024
1.028
1.032
1.037
1.040
Table obtained from Lange's Handbook of Chemistry , 10th ed. Specific gravity is referenced to water at 15.6 °C.
See also "Typical Freezing and Boiling Points of Aqueous Solutions of DOWTHERM SR-1 and DOWTHERM-SR4000" (PDF). Dow Chemical. Archived from the original (PDF) on 27 September 2007. Retrieved 13 June 2007.
Distillation data
Vapor–liquid equilibrium for ethylene glycol/water P = 760 mmHg
BP
% by mole water
liquid
vapor
110.00
79.8
99.3
116.40
61.3
98.5
124.30
55.9
97.7
124.50
55.3
97.6
126.00
48.2
97.1
128.10
42.6
96.3
129.50
41.1
96.2
130.50
38.8
95.5
131.20
36.5
95.2
135.20
28.9
92.6
136.00
28.3
92.4
138.00
24.1
90.9
142.50
21.6
88.7
149.00
17.8
85.2
158.10
12.9
77.6
167.40
10.2
70.6
178.60
6.5
56.3
184.20
3.4
37.9
Vapor–liquid equilibrium for ethylene glycol/methanol P = 760 mmHg
BP
% by mole methanol
liquid
vapor
66.70
93.0
99.9
73.20
82.1
99.8
79.60
66.4
99.7
84.70
53.0
99.3
90.20
45.7
99.0
93.80
40.6
98.5
101.40
36.3
97.9
102.70
35.6
97.5
104.90
32.2
96.7
105.10
22.7
94.6
109.90
21.2
93.3
113.00
19.5
92.3
121.50
13.7
86.4
149.60
10.3
74.6
157.50
4.6
51.5
166.30
3.6
42.0
175.20
2.5
30.4
183.50
1.4
18.1
189.10
0.5
6.8
Spectral data
This box:
References
^ David R. Lide. Handbook of chemistry and physics CRC (2007) , 87th ed.
"Temperature Dependent Properties. [PVP] Vapor pressure of ETHYLENE GLYCOL" (Queriable database). Pure Component Properties . Chemical Engineering Research Information Center. Retrieved 14 May 2007.^ "Binary Vapor–Liquid Equilibrium Data" (Queriable database). Chemical Engineering Research Information Center. Retrieved 8 June 2007.
Category :
Ethylene glycol (data page)
Add topic
Text is available under the Creative Commons Attribution-ShareAlike License. Additional terms may apply.
**DISCLAIMER** We are not affiliated with Wikipedia, and Cloudflare.
The information presented on this site is for general informational purposes only and does not constitute medical advice.
You should always have a personal consultation with a healthcare professional before making changes to your diet, medication, or exercise routine.
AI helps with the correspondence in our chat.
We participate in an affiliate program. If you buy something through a link, we may earn a commission 💕
↑
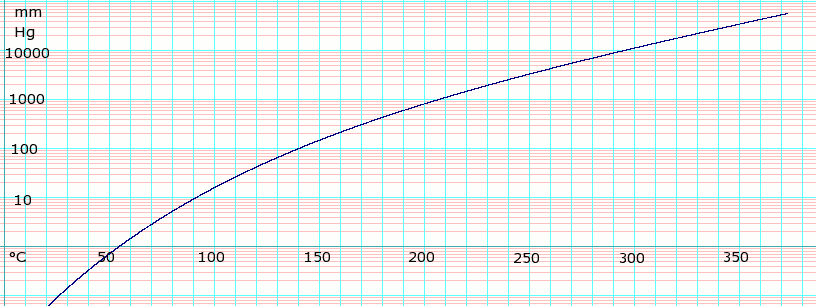
 obtained from CHERIC
obtained from CHERIC