 | |
 | |
 | |
 | |
| Names | |
|---|---|
| IUPAC name Bismuth(III) iodide | |
| Other names Bismuth iodide, bismuth triiodide | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| ChemSpider | |
| ECHA InfoCard | 100.029.207 |
| EC Number |
|
| PubChem CID | |
| UNII | |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | BiI3 |
| Molar mass | 589.69 g/mol |
| Appearance | Greenish-black crystals |
| Density | 5.778 g/cm |
| Melting point | 408.6 °C (767.5 °F; 681.8 K) |
| Boiling point | 542 °C (1,008 °F; 815 K) |
| Solubility in water | 0.7761 mg/100 mL (20 °C) |
| Solubility product (Ksp) | 7.71×10 |
| Solubility | 50 g/100 mL ethanol 50 g/100 mL 2 M hydrochloric acid |
| Magnetic susceptibility (χ) | −200.5·10 cm/mol |
| Structure | |
| Crystal structure | Trigonal, hR24 |
| Space group | R-3, No. 148 |
| Hazards | |
| GHS labelling: | |
| Pictograms | 
|
| Signal word | Danger |
| Hazard statements | H314 |
| Precautionary statements | P260, P264, P280, P301+P330+P331, P303+P361+P353, P304+P340, P305+P351+P338, P310, P321, P363, P405, P501 |
| NFPA 704 (fire diamond) |
 |
| Related compounds | |
| Other anions | Bismuth(III) fluoride Bismuth(III) chloride Bismuth(III) bromide |
| Other cations | Nitrogen triiodide Phosphorus triiodide Antimony triiodide |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa). Infobox references | |
Bismuth(III) iodide is the inorganic compound with the formula BiI3. This gray-black salt is the product of the reaction of bismuth and iodine, which once was of interest in qualitative inorganic analysis.
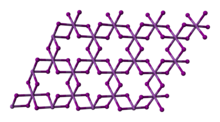
Bismuth(III) iodide adopts a distinctive crystal structure, with iodide centres occupying a hexagonally closest-packed lattice, and bismuth centres occupying either none or two-thirds of the octahedral holes (alternating by layer), therefore it is said to occupy one third of the total octahedral holes.
Synthesis
Bismuth(III) iodide forms upon heating an intimate mixture of iodine and bismuth powder:
- 2Bi + 3I2 → 2BiI3
BiI3 can also be made by the reaction of bismuth oxide with aqueous hydroiodic acid:
- Bi2O3(s) + 6HI(aq) → 2BiI3(s) + 3H2O(l)
Reactions
Since bismuth(III) iodide is insoluble in water, an aqueous solution can be tested for the presence of Bi ions by adding a source of iodide such as potassium iodide. A black precipitate of bismuth(III) iodide indicates a positive test.
Bismuth(III) iodide forms iodobismuth(III) anions when heated with halide donors:
- 2 NaI + BiI3 → Na2
Bismuth(III) iodide catalyzes the Mukaiyama aldol reaction. Bi(III) is also used in a Barbier type allylation of carbonyl compounds in combination with a reducing agent such as zinc or magnesium.
References
- John Rumble (June 18, 2018). CRC Handbook of Chemistry and Physics (99 ed.). CRC Press. pp. 5–188. ISBN 978-1138561632.
- Norman, Nicholas C. (1998), Chemistry of Arsenic, Antimony and Bismuth, Springer, p. 95, ISBN 0-7514-0389-X, retrieved 2008-06-03
- "Bismuth iodide", McGraw-Hill Dictionary of Scientific and Technical Terms, McGraw-Hill, 2003, retrieved 2008-06-19
- Turner, Jr., Francis M.; Berolzheimer, Daniel D.; Cutter, William P.; Helfrich, John (1920), The Condensed Chemical Dictionary, New York: Chemical Catalog Company, p. 107, retrieved 2008-06-19
- Smart, Lesley; Moore, Elaine A. (2005), Solid State Chemistry: An Introduction, CRC Press, p. 40, ISBN 0-7487-7516-1, retrieved 2008-06-19
- Mackay, Rosemary Ann; Henderson, W. (2002), Introduction to Modern Inorganic Chemistry, CRC Press, pp. 122–6, ISBN 0-7487-6420-8, retrieved 2008-06-19
- Watt, George W.; Hakki, Wafai W.; Choppin, Gregory R. (1953). "Bismuth(III) Iodide". Inorganic Syntheses. Inorganic Syntheses. Vol. 4. pp. 114–116. doi:10.1002/9780470132357.ch38. ISBN 978-0-470-13163-3.
- Erdmann, Hugo; Dunlap, Frederick Leavy (1900), Handbook of Basic Tables for Chemical Analysis, New York: John Wiley & Sons, p. 76, retrieved 2008-06-19
- Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. p. 559. ISBN 978-0-08-037941-8.
- Bruno, Thomas J.; Svoronos, Paris D. N. (2003), Handbook of Basic Tables for Chemical Analysis, CRC Press, p. 549, ISBN 0-8493-1573-5, retrieved 2008-06-19
- Norman, Nicholas C. (1998), Chemistry of Arsenic, Antimony and Bismuth, Springer, pp. 168–70, ISBN 0-7514-0389-X, retrieved 2008-06-19
| Bismuth compounds | |||
|---|---|---|---|
| Bismuth(III) |
| ||
| Bismuth(V) |
| ||
| Salts and covalent derivatives of the iodide ion | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||