| |||
| Names | |||
|---|---|---|---|
| Other names (2S,3S)-2-amino-3-hydroxybutanoic acid | |||
| Identifiers | |||
| CAS Number |
| ||
| 3D model (JSmol) |
| ||
| ChEBI |
| ||
| ChEMBL |
| ||
| ChemSpider | |||
| EC Number |
| ||
| KEGG |
| ||
| PubChem CID |
| ||
| UNII |
| ||
| CompTox Dashboard (EPA) |
| ||
InChI
| |||
SMILES
| |||
| Properties | |||
| Chemical formula | C4H9NO3 | ||
| Molar mass | 119.120 g·mol | ||
| Appearance | White solid | ||
| Melting point | 273.5–275.0 °C (524.3–527.0 °F; 546.6–548.1 K) decomposition | ||
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa). Infobox references | |||
Allothreonine is an amino acid with the formula CH3CH(OH)CH(NH2)CO2H. It is the diastereomer of the amino acid threonine. Like most other amino acids, allothreonine is a water-soluble colorless solid. Although not one of the proteinogenic amino acids, it has often been the subject for the synthesis of novel proteins using an expanded genetic code. Racemic allothreonine can be produced in the laboratory from bromomethoxybutyric acid.
Structure
Threonine has R, S stereochemistry at carbons 2 and 3 for the naturally occurring stereoisomer and S, R stereochemistry for its enantiomer. Allothreonine has S, S stereochemistry at carbons 2 and 3 in the natural stereoisomer, but R, R in the very rare enantiomer.
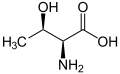  |
| L-Threonine (2S,3R) and D-Threonine (2R,3S) |
 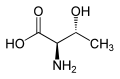 |
| L-Allothreonine (2S,3S) and D-Allothreonine (2R,3R) |
Occurrence
Katanosins are a group of potent antibiotics contain allothreonine.
Peptides containing the allothreonine residue have also been isolated from natural source.
References
- Johnson, Brooke A.; Clark, Kenzie A.; Bushin, Leah B.; Spolar, Calvin N.; Seyedsayamdost, Mohammad R. (2024). "Expanding the Landscape of Noncanonical Amino Acids in RiPP Biosynthesis". Journal of the American Chemical Society. 146 (6): 3805–3815. doi:10.1021/jacs.3c10824. PMID 38316431.
- Carter, Herbert E.; West, Harold D. (1940). "dl-Threonine". Organic Syntheses. 20: 101. doi:10.15227/orgsyn.020.0101.
- Bonner, DP; O'Sullivan, J; Tanaka, SK; Clark, JM; Whitney, RR (1988). "Lysobactin, a Novel Antibacterial Agent Produced by Lysobacter sp. II. Biological Properties". The Journal of Antibiotics. 41 (12): 1745–51. doi:10.7164/antibiotics.41.1745. PMID 3209466.
- Sarabia, Francisco; Chammaa, Samy; García-Ruiz, Cristina (2011). "Solid Phase Synthesis of Globomycin and SF-1902 A5". The Journal of Organic Chemistry. 76 (7): 2132–2144. doi:10.1021/jo1025145. PMID 21366318.