 | |
 | |
| Names | |
|---|---|
| Preferred IUPAC name 3-Ethyl-1-methyl-3H-imidazol-1-ium chloride | |
| Other names Cl | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.129.917 |
| EC Number |
|
| PubChem CID | |
| UNII | |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | C6H11ClN2 |
| Molar mass | 146.62 g·mol |
| Melting point | 77 to 79 °C (171 to 174 °F; 350 to 352 K) |
| Hazards | |
| GHS labelling: | |
| Pictograms | 
|
| Signal word | Warning |
| Hazard statements | H302, H315, H319 |
| Precautionary statements | P264, P270, P280, P301+P312, P302+P352, P305+P351+P338, P321, P330, P332+P313, P337+P313, P362, P501 |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |
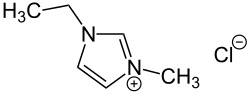
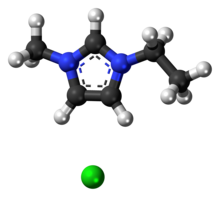
1-Ethyl-3-methylimidazolium chloride or Cl is an ionic liquid that can be used in cellulose processing. The cation consists of a five-membered ring with two nitrogen and three carbon atoms, i.e. a derivative of imidazole, with ethyl and methyl groups substituted at the two nitrogen atoms. Its melting point is 77–79 °C.
References
- Scientists Propose a More Efficient Way to Make Ethanol, The New York Times, March 2, 2010
- Joseph B. Binder and Ronald T. Raines (2010). "Fermentable sugars by chemical hydrolysis of biomass" (PDF). PNAS. 107 (10): 4516–4521. doi:10.1073/pnas.0912073107. PMC 2842027. PMID 20194793.
- 1-Ethyl-3-methylimidazolium chloride, chemexper.com
- MSDS
This article about a heterocyclic compound is a stub. You can help Misplaced Pages by expanding it. |