| |||
| Names | |||
|---|---|---|---|
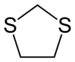
| IUPAC name 1,3-Dithiolane | |||
| Identifiers | |||
| CAS Number | |||
| 3D model (JSmol) | |||
| Beilstein Reference | 102455 | ||
| ChEBI | |||
| ChemSpider | |||
| Gmelin Reference | 82036 | ||
| PubChem CID | |||
| UNII | |||
| CompTox Dashboard (EPA) | |||
InChI
| |||
SMILES
| |||
| Properties | |||
| Chemical formula | C3H6S2 | ||
| Molar mass | 106.20 g·mol | ||
| Related compounds | |||
| Related compounds | Ethane-1,2-dithiol | ||
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa). Infobox references | |||
1,3-Dithiolane is the organosulfur compound with the formula CH2S2C2H4. Also classified as a heterocycle related cyclopentane by replacing two methylene bridges (-CH
2- units) with thioether groups. It is an isomer of 1,2-dithiolane. 1,3-Dithiolanes are compounds where one or more H atoms of the parent 1,3-dithiolane are replaced by other groups. These species are more widely studied.
Synthesis
A common family of 1,3-dithiolanes have the formula RCHS2C2H4. They are obtained by treating an aldehyde with 1,2-ethanedithiol. Related compounds with the formula R2CS2C2H4 are obtained by condensation of 1,2-ethanedithiol with ketones. The dithiolane protected aldehydes and ketones are amenable to many reactions without perturbing the dithiolane ring.

Dithiolanes can often be reverted, i.e., deprotected, to the parent aldehyde and ketone. A variety of reagents have been developed for that purpose.
Reactions
1,3-Dithiolanes derived from aldehydes can be deprotonated:
- RCHS2C2H4 + BuLi → RCLiS2C2H4 + BuH
These organolithium compounds degrade with loss of ethylene to give the dthiocarboxylate:
- RCLiS2C2H4 → RCS2Li + C2H4
In contrast, 2-lithio-1,3-dithianes (RCLiS2C3H6) are long-lived.
1,3-Dithiolanes are susceptible to a variety of degradation processes involving organometallic reagents leading to other organosulfur compounds.
References
- Teuber, Lene (1990). "Naturally Occurring 1,2-Dithiolanes and 1,2,3-Trithianes. Chemical and Biological Properties". Sulfur Reports. 9 (4): 257–333. doi:10.1080/01961779008048732.
- ^ Ni, Zhi-Jie; Luh, Tien-Yau (1992). "Nickel-Catalyzed Silylolefination of Allylic Dithioacetals: (E,E)-Trimethyl(4-Phenyl-1,3-Butadienyl)Silane". Organic Syntheses. 70: 240. doi:10.15227/orgsyn.070.0240.
- ^ Wilson, S. R.; Georgiadis, G. M. (1983). "Mercaptans from Thioketals: Cyclododecyl Mercaptan". Organic Syntheses. 61: 74. doi:10.15227/orgsyn.061.0074.
- Dahnke, Karl R.; Paquette, Leo A. (1993). "2-Methylene-1,3-Dithiolane". Organic Syntheses. 71: 175. doi:10.15227/orgsyn.071.0175.
- Wuts, P. G. M.; Greene, T. W. (2006). Greene's Protective Groups in Organic Synthesis. NY: J. Wiley. doi:10.1002/0470053488. ISBN 9780470053485. S2CID 83393227.
- Banerjee, Ajoy K.; Laya, M. S. (2000). "Reagents for the preparation and cleavage of 1,3-dithiolanes". Russian Chemical Reviews. 69 (11): 947–955. Bibcode:2000RuCRv..69..947B. doi:10.1070/rc2000v069n11abeh000583. S2CID 250918297.