This is an old revision of this page, as edited by Evolution and evolvability (talk | contribs) at 01:11, 9 June 2018 (→Target: update from v:WikiJournal_of_Science/ShK_toxin:_history,_structure_and_therapeutic_applications_for_autoimmune_diseases). The present address (URL) is a permanent link to this revision, which may differ significantly from the current revision.
Revision as of 01:11, 9 June 2018 by Evolution and evolvability (talk | contribs) (→Target: update from v:WikiJournal_of_Science/ShK_toxin:_history,_structure_and_therapeutic_applications_for_autoimmune_diseases)(diff) ← Previous revision | Latest revision (diff) | Newer revision → (diff) Protein domain| ShK domain-like | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
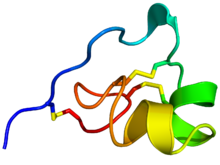 Rainbow colored cartoon diagram (N-terminus = blue, C-terminus = red) of an NMR solution structure of the ShK toxin. Sidechains of cysteine residues involved in disulfide linkages are displayed as sticks and the sulfur atoms in these links are colored yellow. Rainbow colored cartoon diagram (N-terminus = blue, C-terminus = red) of an NMR solution structure of the ShK toxin. Sidechains of cysteine residues involved in disulfide linkages are displayed as sticks and the sulfur atoms in these links are colored yellow. | |||||||||||
| Identifiers | |||||||||||
| Symbol | ShK | ||||||||||
| Pfam | PF01549 | ||||||||||
| InterPro | IPR003582 | ||||||||||
| SMART | SM00254 | ||||||||||
| SCOP2 | 1roo / SCOPe / SUPFAM | ||||||||||
| TCDB | 8.B.14 | ||||||||||
| OPM superfamily | 475 | ||||||||||
| OPM protein | 2lg4 | ||||||||||
| |||||||||||
Stichodactyla toxin (ShK) is a 35-residue basic peptide from the sea anemone Stichodactyla helianthus that blocks a number of potassium channels. An analogue of ShK called ShK-186 or Dalazatide is in human trials as a therapeutic for autoimmune diseases.
History
Stichodactyla helianthus is a species of sea anemone (Phylum: Cnidaria) belonging to the family Stichodactylidae. Helianthus comes from the Greek words Helios meaning sun, and anthos meaning flower, which corresponds to S. helianthus common name "sun anemone". It is sessile and uses potent neurotoxins for defense against its primary predator, the spiny lobster. The venom contains, among other components, numerous ion channel-blocking peptides. In 1995, a group led by Olga Castaneda and Evert Karlsson isolated ShK, a potassium channel-blocking 35-residue peptide from S. helianthus. The same year, William Kem and his collaborator Michael Pennington synthesized and folded ShK, and showed it blocked neuronal and lymphocyte voltage-dependent potassium channels. In 1996, Ray Norton determined the three-dimensional structure of ShK. In 2005-2006, George Chandy, Christine Beeton and Michael Pennington developed ShK-170 and ShK-186, selective blockers of Kv1.3. ShK-186, now called Dalazatide, was advanced to human trials in 2015-2017 by Shawn Iadonato and Eric Tarcha, as the first-in-man Kv1.3 blocker for autoimmune disease.References used only in figure 1
Structure
ShK is cross-linked by three disulfide bridges: Cys3-Cys35, Cys12-Cys28, and Cys17-Cys32. The solution structure of ShK reveals two short α-helices comprising residues 14-19 and 21-24; the N-terminal eight residues adopt an extended conformation, followed by a pair of interlocking turns that resemble a 310 helix; the C-terminal Cys35 residue forms a nearly head-to-tail cyclic structure through a disulfide bond with Cys3.

Phylogenetic relationships of ShK and ShK domains
ShK is cross-linked by three disulfide bridges: Cys3-Cys35, Cys12-Cys28, and Cys17-Cys32 (Figure 2). The solution structure of ShK reveals two short α-helices comprising residues 14-19 and 21-24; the N-terminal eight residues adopt an extended conformation, followed by a pair of interlocking turns that resemble a 310 helix; the C-terminal Cys35 residue forms a nearly head-to-tail cyclic structure through a disulfide bond with Cys3. Figure 3 shows the three-dimensional structure of ShK with key residues, in addition to the structures of related peptides: MMP-23’s ShK domain, BmK1 (from the filarial worm), and ShK-192 (an analogue of ShK-186), and the homology model of ShK-EWSS.
Phylogenetic relationships of ShK and ShK domains
The SMART database at the EMBL, as of May 2018, lists 3345 protein domains with structural resemblance to ShK in 1797 proteins (1 to 8 domains/protein), many in the worm Caenorhabditis elegans and venomous snakes. The majority of these domains are in metallopeptidases, whereas others are in prolyl 4-hydroxylases, tyrosinases, peroxidases, oxidoreductases, or proteins containing epidermal growth factor-like domains, thrombospondin-type repeats, or trypsin-like serine protease domains. The only human proteins containing ShK-like domains are MMP-23 (matrix metalloprotease 23) and MFAP-2 (microfibril-associated glycoprotein 2).
Channel targets
The ShK peptide blocks potassium (K) ion channels Kv1.1, Kv1.3, Kv1.6, Kv3.2 and KCa3.1 with nanomolar to picomolar potency, and has no effect on the HERG (Kv11.1) cardiac potassium channel. The neuronal Kv1.1 channel and the T lymphocyte Kv1.3 channel are most potently inhibited by ShK.
ShK binding configuration in K channels
ShK and its analogues are blockers of the channel pore. They bind to all four subunits in the K channel tetramer by interacting with the shallow vestibule at the outer entrance to the channel pore. These peptides are anchored in the external vestibule by two key interactions. The first is Lys22, which protrudes into and occludes the channel’s pore like a "cork in a bottle" and blocks the passage of potassium ions through the channel pore. The second is the neighboring Tyr23, which together with Lys22 forms a “functional dyad” required for channel block. Many K channel-blocking peptides contain such a dyad of a lysine and a neighboring aromatic or aliphatic residue. Some K channel-blocking peptides lack the functional dyad, but even in these peptides a lysine physically blocks the channel, regardless of the position of the lysine in the peptide sequence. Additional interactions anchor ShK and its analogues in the external vestibule and contribute to potency and selectivity. For example, Arg11 and Arg29 in ShK interact with two Asp386 residues in adjacent subunits in the mouse Kv1.3 external vestibule (corresponds to Asp433 in human Kv1.3).
| Channel | ShK(IC50) | ShK-186
(IC50) | ShK-192 (IC50) | ShK-EWSS
(IC50) | ShK-F6CA(IC50) | ShK-198(IC50) | MMP-23 ShK domain(IC50) |
| Kv1.1 | 16-28 pM | 7 nM | 22 nM | 5.4 nM | 4 nM | 159 pM | 49 μM |
| Kv1.2 | 10 nM | 48 nM | ND | >100 nM | >100 nM | ND | >100 μM |
| Kv1.3 | 10-16 pM | 70 pM | 140 pM | 34 pM | 48 pM | 41 pM | 2.8 μM |
| Kv1.6 | 200 pM | 18 nM | 10.6 nM | ND | ND | ND | 400 nM |
| Kv3.2 | 5 nM | 20 nM | 4.2 nM | ND | ND | ND | 49 μM |
| KCa3.1 | 30 nM | 115 nM | >100 nM | >100 nM | ND | ND | >100 μM |
Target
ShK toxin blocks the K channels Kv1.1, Kv1.3, Kv1.6, Kv3.2 and KCa3.1, The peptide binds to all four subunits in the Kv1.3 tetramer through its interaction with the shallow vestibule at the outer entrance of the ion conduction pathway. The peptide's Lysine residue occludes the channel pore like a "cork in a bottle". This blocks the entrance to the pore.
ShK blocks the Kv1.3 channel in T cells with a Kd of about 11 pM. It blocks the neuronal Kv1.1 and Kv1.6 channels with Kds of 16 pM and 200 pM respectively. The Kv3.2 and KCa3.1 channels are more than 1000 times less sensitive to the peptide.
Several ShK analogs have been generated to enhance specificity for the Kv1.3 channel over the Kv1.1, Kv1.6 and Kv3.2 channels. The first analog that showed some degree of specificity was ShK-Dap22. Attaching a fluorescein to the N-terminus of the peptide via a hydrophilic AEEA linker (2-aminoethoxy-2-ethoxy acetic acid; mini-PEG) resulted in a peptide, ShK-F6CA, with 100-fold specificity for Kv1.3 over Kv1.1 and related channels. Based on this surprising finding additional analogs were made. ShK-170 , contains a L-phosphotyrosine in place of the fluorescein in ShK-F6CA. It blocks Kv1.3 with a Kd of 69 pM and shows exquisite specificity for Kv1.3. However, it is chemically unstable. To improve stability a new analog, ShK-186 , was made with the C-terminal carboxyl of ShK-170 replaced by an amide; ShK-186 is otherwise identical to ShK-170. In rats and squirrel monkeys, an indium-labeled ShK-186 analog called ShK-221, was slowly released from the injection site and maintained blood levels above the channel blocking dose for 3–5 days ShK-192 is a new analog with increased stability. It contains norleucine in place of methionine to avoid methionine oxidation, and the terminal phosphotyrosine is replaced by a non-hydrolyzable para-phosphonophenylalanine (Ppa) group. ShK-192 is effective in ameliorating disease in rat models of multiple sclerosis. The D-diastereomer of ShK is also stable but blocks Kv1.3 with 2800-fold potency than the L-form (Kd = 36 nM) and it only exhibits 2-fold specificity for Kv1.3 over Kv1.1. ShK-K-amide is a new analog with a C-terminal lysine. It blocks Kv1.3 with roughly 50-fold greater potency (IC50 of 26 ± 3 pM) than Kv1.1 ( IC50 of 942 ± 120 pM), and suppresses proliferation of human T cells (IC50 ≈ 3 nM).
Kv1.3 and KCa3.1 regulate membrane potential and calcium signaling of T cells. Calcium entry through the CRAC channel is promoted by potassium efflux through the Kv1.3 and KCa3.1 potassium channels. Blockade of Kv1.3 channels in effector-memory T cells by ShK-186 suppresses calcium signaling, cytokine production (interferon-gamma, interleukin 2) and cell proliferation. In vivo, ShK-186 paralyzes effector-memory T cells at the sites of inflammation and prevent their reactivation in inflamed tissues. In contrast, ShK-186 does not affect the homing to and motility within lymph nodes of naive and central memory T cells, most likely because these cells express the KCa3.1 channel and are therefore protected from the effect of Kv1.3 blockade. In proof-of-concept studies, ShK and its analogs have prevented and treated disease in rat models of multiple sclerosis, rheumatoid arthritis, and delayed type hypersensitivity. ShK-186, due to its durable pharmacological action, is effective in ameliorating disease in rat models of delayed type hypersensitivity, multiple sclerosis (experimental autoimmune encephalomyelitis) and rheumatoid arthritis (pristane induced arthritis) when administered once every 2–5 days. ShK-186 has completed non-clinical safety studies. ShK-186 is the subject of an open Investigational New Drug (IND) application in the USA, and has completed human phase 1A and 1B trials in healthy volunteers.
As ShK toxin binds to the synaptosomal membranes, it facilitates an acetylcholine release at avian neuromuscular junctions while the Kv3.2 channels are expressed in neurons that fire at a high frequency (such as cortical GABAergic interneurons), due to their fast activation and deactivation rates. By blocking Kv3.2, ShK toxin depolarises the cortical GABAergic interneurons. Kv3.2 is also expressed in pancreatic beta cells. These cells are thought to play a role in their delayed-rectifier current, which regulates glucose-dependent firing. Therefore, ShK, as a Kv3.2 blocker, might be useful in the treatment of type-2 diabetes, although inhibition of the delayed-rectifier current has not yet been observed in human cells even when very high ShK concentrations were used.
Toxicity
Toxicity of ShK toxin in mice is quite low. The median paralytic dose is about 25 mg/kg bodyweight (which translates to 0.5 mg per 20 g mouse). In rats the therapeutic safety index was greater than 75-fold.
ShK-Dap22 is less toxic, even a dose of 1.0 mg dose did not cause hyperactivity, seizures or mortality. The median paralytic dose was 200 mg/kg body weight.
ShK-170 does not cause significant toxicity in vitro. The peptide was not toxic to human and rat lymphoid cells incubated for 48 h with 100 nM of ShK-170 (>1200 times greater than the Kv1.3 half-blocking dose). The same high concentration of ShK-170 was negative in the Ames test on tester strain TA97A, suggesting that it is not a mutagen. ShK-170 had no effect on heart rate or heart rate variability parameters in either the time or the frequency domain in rats. It does not block the hERG (Kv11.1) channel that is associated with drug-associated cardiac arrhythmias. Repeated daily administration of the peptide by subcutaneous injection (10 µg/kg/day) for 2 weeks to rats does not cause any changes in blood counts, blood chemistry or in the proportion of thymocyte or lymphocyte subsets. Furthermore, the rats administered the peptide gain weight normally.
ShK-186 is also safe. Repeated daily administration by subcutaneous injection of ShK-186 (100 µg/kg/day) for 4 weeks to rats does not cause any changes in blood counts, blood chemistry or histopathology. Furthermore, ShK-186 did not compromise the protective immune response to acute influenza viral infection or acute bacterial (Chlamydia) infection in rats at concentrations that were effective in ameliorating autoimmune diseases in rat models. Interestingly, rats repeatedly administered ShK-186 for a month by subcutaneous injection (500 µg/kg/day) developed low titer anti-ShK antibodies. The reason for the low immunogenicity of the peptide is not well understood. ShK-186 has completed GLP (Good Laboratory Practice) non-clinical safety studies in rodents and non-human primates. ShK-186 (aka Dalazatide) which was licensed to Kineta Bio is the subject of an open Investigational New Drug (IND) application in the United States of America, and has recently completed human phase 1A and 1b trials in healthy volunteers. A second human phase 1b was recently completed in 2015 in psoriasis patients. Dalazatide was shown to significantly ameliorate symptoms in 90% patients with active plaque psoriasis with a 60 mcg weekly dose.
Many groups are developing Kv1.3 blockers for the treatment of autoimmune diseases.
Use
Because ShK toxin is a specific inhibitor of Kv1.1, Kv1.3, Kv1.6, Kv3.2 and KCa3.1, it may serve as a useful pharmacological tool for studying these channels. The Kv1.3 specific ShK analogs, ShK-170, ShK-186 and ShK-192, have been demonstrated to be effective in rat models of autoimmune diseases, and these or related analogs might have use as therapeutics for human autoimmune diseases.
Kv1.3 is also considered a therapeutic target for the treatment of obesity, for enhancing peripheral insulin sensitivity in patients with type-2 diabetes mellitus, and for preventing bone resorption in periodontal disease. Furthermore, because pancreatic beta cells, which have Kv3.2 channels, are thought to play a role in glucose-dependent firing, ShK, as a Kv3.2 blocker, might be useful in the treatment of type-2 diabetes, although inhibition of the delayed-rectifier current has not yet been observed in human cells even when very high ShK concentrations were used.
References
- Cite error: The named reference
Tudor_1996was invoked but never defined (see the help page). - Norton, Raymond; Pennington, Michael; Wulff, Heike (2004-12-01). "Potassium Channel Blockade by the Sea Anemone Toxin ShK for the Treatment of Multiple Sclerosis and Other Autoimmune Diseases". Current Medicinal Chemistry. 11 (23): 3041–3052. doi:10.2174/0929867043363947. ISSN 0929-8673.
- Castañeda, O; Sotolongo, V; Amor, AM; Stöcklin, R; Anderson, AJ; Harvey, AL; Engström, A; Wernstedt, C; Karlsson, E (1995). "Characterization of a potassium channel toxin from the Caribbean Sea anemone Stichodactyla helianthus". Toxicon. 33 (5): 603-613. doi:10.1016/0041-0101(95)00013-C.
- Pennington, M; Byrnes, ME; Zaydenberg, I; Khaytin, I; de Chastonay, J; Krafte, DS; Hill, R; Mahnir, VM; Volberg, WA; Gorczyca, W (1995). "Chemical synthesis and characterization of ShK toxin: a potent potassium channel inhibitor from a sea anemone". International Journal of Peptide and Protein Research. 46 (5): 354-358. doi:10.1111/j.1399-3011.1995.tb01068.x.
{{cite journal}}: Unknown parameter|month=ignored (help) - Tudor, JE; Pallaghy, PK; Pennington, W; Norton, RS (1996). "Solution structure of ShK toxin, a novel potassium channel inhibitor from a sea anemone". Nature Structural & Molecular Biology. 3: 317–320. doi:10.1038/nsb0496-317.
{{cite journal}}: Unknown parameter|month=ignored (help) - Beeton, C; Pennington, MW; Wulff, H; Singh, S; Nugent, D; Crossley, G; Khaytin, I; Calabresi, PA; Chen, CY; Gutman, GA; Chandy, KG (2005). "Targeting effector memory T cells with a selective peptide inhibitor of Kv1.3 channels for therapy of autoimmune diseases". Molecular Pharmacology. 67 (4): 1369-1381. doi:10.1124/mol.104.008193.
{{cite journal}}: Unknown parameter|month=ignored (help) - Beeton, C; Wulff, H; Standifer, NE; Azam, P; Mullen, KM; Pennington, MW; Kolski-Andreaco, A; Wei, E; Grino, A; Counts, DR; Wang, PH; LeeHealey, CJ; Andrews, BS; Sankaranarayanan, A; Homerick, D; Roeck, WW; Tehranzadeh, J; Stanhope, KL; Zimin, P; Havel, PJ; Griffey, S; Knaus, HG; Nepom, GT; Gutman, GA; Calabresi, PA; Chandy, KG (2006). "Kv1.3 channels are a therapeutic target for T cell-mediated autoimmune diseases". Proceedings of the National Academy of Sciences of the United States of America. 103 (46): 17414–17419. doi:10.1073/pnas.0605136103.
{{cite journal}}: Unknown parameter|month=ignored (help) - Tarcha, Eric J.; Olsen, Chelsea M.; Probst, Peter; Peckham, David; Muñoz-Elías, Ernesto J.; Kruger, James G.; Iadonato, Shawn P. (2017-07-19). "Safety and pharmacodynamics of dalazatide, a Kv1.3 channel inhibitor, in the treatment of plaque psoriasis: A randomized phase 1b trial". PLOS ONE. 12 (7): e0180762. doi:10.1371/journal.pone.0180762. ISSN 1932-6203. PMC 5516987. PMID 28723914.
{{cite journal}}: CS1 maint: PMC format (link) CS1 maint: unflagged free DOI (link) - "New toxins from marine organisms". Toxicon. 29 (10): 1168. 1991. doi:10.1016/0041-0101(91)90154-j. ISSN 0041-0101.
- Harvey, A (1991). "Dendrotoxin-like activity isolated from sea anemones". British Journal of Pharmacology. 104 (Suppl): Poster 34. ISSN 0007-1188. PMC 1907833. PMID 1912993.
{{cite journal}}: CS1 maint: PMC format (link) - Pennington, M.W.; Mahnir, V.M.; Krafte, D.S.; Zaydenberg, I.; Byrnes, M.E.; Khaytin, I.; Crowley, K.; Kem, W.R. (1996). "Identification of Three Separate Binding Sites on SHK Toxin, a Potent Inhibitor of Voltage-Dependent Potassium Channels in Human T-Lymphocytes and Rat Brain". Biochemical and Biophysical Research Communications. 219 (3): 696–701. doi:10.1006/bbrc.1996.0297. ISSN 0006-291X.
- Dauplais, M.; Lecoq, A.; Song, J.; Cotton, J.; Jamin, N.; Gilquin, B.; Roumestand, C.; Vita, C.; de Medeiros, C. L. (1997). "On the convergent evolution of animal toxins. Conservation of a diad of functional residues in potassium channel-blocking toxins with unrelated structures". The Journal of Biological Chemistry. 272 (7): 4302–4309. ISSN 0021-9258. PMID 9020148.
- Tudor, Jane E.; Pennington, Michael W.; Norton, Raymond S. (1998). "Ionisation behaviour and solution properties of the potassium-channel blocker ShK toxin". European Journal of Biochemistry. 251 (1–2): 133–141. doi:10.1046/j.1432-1327.1998.2510133.x. ISSN 0014-2956.
- Pennington, Michael W.; Lanigan, Mark D.; Kalman, Katalin; Mahnir, Vladimir M.; Rauer, Heiko; McVaugh, Cheryl T.; Behm, David; Donaldson, Denise; Chandy, K. George (1999). "Role of Disulfide Bonds in the Structure and Potassium Channel Blocking Activity of ShK Toxin†". Biochemistry. 38 (44): 14549–14558. doi:10.1021/bi991282m. ISSN 0006-2960.
- Lanigan, Mark D.; Tudor, Jane E.; Pennington, Michael W.; Norton, Raymond S. (2001). "A helical capping motif in ShK toxin and its role in helix stabilization". Biopolymers. 58 (4): 422–436. ISSN 0006-3525.
- Baell, Jonathan B.; Harvey, Andrew J.; Norton, Raymond S. (2002). "Design and synthesis of type-III mimetics of ShK toxin". Journal of Computer-Aided Molecular Design. 16 (4): 245–262. doi:10.1023/a:1016310602813. ISSN 0920-654X.
- Chang, Shih Chieh; Galea, Charles A.; Leung, Eleanor W.W.; Tajhya, Rajeev B.; Beeton, Christine; Pennington, Michael W.; Norton, Raymond S. (2012). "Expression and isotopic labelling of the potassium channel blocker ShK toxin as a thioredoxin fusion protein in bacteria". Toxicon. 60 (5): 840–850. doi:10.1016/j.toxicon.2012.05.017. ISSN 0041-0101.
- Dang, Bobo; Kubota, Tomoya; Mandal, Kalyaneswar; Bezanilla, Francisco; Kent, Stephen B. H. (2013). "Native Chemical Ligation at Asx-Cys, Glx-Cys: Chemical Synthesis and High-Resolution X-ray Structure of ShK Toxin by Racemic Protein Crystallography". Journal of the American Chemical Society. 135 (32): 11911–11919. doi:10.1021/ja4046795. ISSN 0002-7863.
- Lioudyno, Maria I.; Birch, Alexandra M.; Tanaka, Brian S.; Sokolov, Yuri; Goldin, Alan L.; Chandy, K. George; Hall, James E.; Alkire, Michael T. (2013). "Shaker-Related Potassium Channels in the Central Medial Nucleus of the Thalamus Are Important Molecular Targets for Arousal Suppression by Volatile General Anesthetics". Journal of Neuroscience. 33 (41): 16310–16322. doi:10.1523/jneurosci.0344-13.2013. PMC 3792466. PMID 24107962.
{{cite journal}}: CS1 maint: PMC format (link) - Chhabra, Sandeep; Chang, Shih Chieh; Nguyen, Hai M.; Huq, Redwan; Tanner, Mark R.; Londono, Luz M.; Estrada, Rosendo; Dhawan, Vikas; Chauhan, Satendra (2014). "Kv1.3 channel-blocking immunomodulatory peptides from parasitic worms: implications for autoimmune diseases". The FASEB Journal. 28 (9): 3952–3964. doi:10.1096/fj.14-251967. ISSN 0892-6638.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - Zhao, Ruiming; Dai, Hui; Mendelman, Netanel; Cuello, Luis G.; Chill, Jordan H.; Goldstein, Steve A. N. (2015). "Designer and natural peptide toxin blockers of the KcsA potassium channel identified by phage display". Proceedings of the National Academy of Sciences. 112 (50): E7013 – E7021. doi:10.1073/pnas.1514728112. ISSN 0027-8424.
- Tudor, JE; Pallaghy, PK; Pennington, W; Norton, RS (1996). "Solution structure of ShK toxin, a novel potassium channel inhibitor from a sea anemone". Nature Structural & Molecular Biology. 3: 317–320. doi:10.1038/nsb0496-317.
{{cite journal}}: Unknown parameter|month=ignored (help) - Kalman, K.; Pennington, M. W.; Lanigan, M. D.; Nguyen, A.; Rauer, H.; Mahnir, V.; Paschetto, K.; Kem, W. R.; Grissmer, S. (1998-12-04). "ShK-Dap22, a potent Kv1.3-specific immunosuppressive polypeptide". The Journal of Biological Chemistry. 273 (49): 32697–32707. ISSN 0021-9258. PMID 9830012.
- Pennington, M. W.; Beeton, C.; Galea, C. A.; Smith, B. J.; Chi, V.; Monaghan, K. P.; Garcia, A.; Rangaraju, S.; Giuffrida, A. (2009-4). "Engineering a Stable and Selective Peptide Blocker of the Kv1.3 Channel in T Lymphocytes". Molecular Pharmacology. 75 (4): 762–773. doi:10.1124/mol.108.052704. ISSN 0026-895X. PMC 2684922. PMID 19122005.
{{cite journal}}: Check date values in:|date=(help)CS1 maint: PMC format (link) - Pennington, Michael W.; Harunur Rashid, M.; Tajhya, Rajeev B.; Beeton, Christine; Kuyucak, Serdar; Norton, Raymond S. (2012-10-09). "A C-terminally amidated analogue of ShK is a potent and selective blocker of the voltage-gated potassium channel Kv1.3". FEBS Letters. 586 (22): 3996–4001. doi:10.1016/j.febslet.2012.09.038. ISSN 0014-5793. PMC 3496055. PMID 23063513.
{{cite journal}}: CS1 maint: PMC format (link) - Murray, Justin K.; Qian, Yi-Xin; Liu, Benxian; Elliott, Robin; Aral, Jennifer; Park, Cynthia; Zhang, Xuxia; Stenkilsson, Michael; Salyers, Kevin (2015-08-31). "Pharmaceutical Optimization of Peptide Toxins for Ion Channel Targets: Potent, Selective, and Long-Lived Antagonists of Kv1.3". Journal of Medicinal Chemistry. 58 (17): 6784–6802. doi:10.1021/acs.jmedchem.5b00495. ISSN 0022-2623.
- Chang, Shih C.; Huq, Redwan; Chhabra, Sandeep; Beeton, Christine; Pennington, Michael W.; Smith, Brian J.; Norton, Raymond S. (2015-04-23). "N-terminally extended analogues of the K+ channel toxin from Stichodactyla helianthusas potent and selective blockers of the voltage-gated potassium channel Kv1.3". FEBS Journal. 282 (12): 2247–2259. doi:10.1111/febs.13294. ISSN 1742-464X. PMC 4472561. PMID 25864722.
{{cite journal}}: no-break space character in|title=at position 75 (help)CS1 maint: PMC format (link) - Tudor, JE; Pallaghy, PK; Pennington, W; Norton, RS (1996). "Solution structure of ShK toxin, a novel potassium channel inhibitor from a sea anemone". Nature Structural & Molecular Biology. 3: 317–320. doi:10.1038/nsb0496-317.
{{cite journal}}: Unknown parameter|month=ignored (help) - Kalman, K.; Pennington, M. W.; Lanigan, M. D.; Nguyen, A.; Rauer, H.; Mahnir, V.; Paschetto, K.; Kem, W. R.; Grissmer, S. (1998-12-04). "ShK-Dap22, a potent Kv1.3-specific immunosuppressive polypeptide". The Journal of Biological Chemistry. 273 (49): 32697–32707. ISSN 0021-9258. PMID 9830012.
- Pennington, M. W.; Beeton, C.; Galea, C. A.; Smith, B. J.; Chi, V.; Monaghan, K. P.; Garcia, A.; Rangaraju, S.; Giuffrida, A. (2009-4). "Engineering a Stable and Selective Peptide Blocker of the Kv1.3 Channel in T Lymphocytes". Molecular Pharmacology. 75 (4): 762–773. doi:10.1124/mol.108.052704. ISSN 0026-895X. PMC 2684922. PMID 19122005.
{{cite journal}}: Check date values in:|date=(help)CS1 maint: PMC format (link) - Pennington, Michael W.; Harunur Rashid, M.; Tajhya, Rajeev B.; Beeton, Christine; Kuyucak, Serdar; Norton, Raymond S. (2012-10-09). "A C-terminally amidated analogue of ShK is a potent and selective blocker of the voltage-gated potassium channel Kv1.3". FEBS Letters. 586 (22): 3996–4001. doi:10.1016/j.febslet.2012.09.038. ISSN 0014-5793. PMC 3496055. PMID 23063513.
{{cite journal}}: CS1 maint: PMC format (link) - Murray, Justin K.; Qian, Yi-Xin; Liu, Benxian; Elliott, Robin; Aral, Jennifer; Park, Cynthia; Zhang, Xuxia; Stenkilsson, Michael; Salyers, Kevin (2015-08-31). "Pharmaceutical Optimization of Peptide Toxins for Ion Channel Targets: Potent, Selective, and Long-Lived Antagonists of Kv1.3". Journal of Medicinal Chemistry. 58 (17): 6784–6802. doi:10.1021/acs.jmedchem.5b00495. ISSN 0022-2623.
- Chang, Shih C.; Huq, Redwan; Chhabra, Sandeep; Beeton, Christine; Pennington, Michael W.; Smith, Brian J.; Norton, Raymond S. (2015-04-23). "N-terminally extended analogues of the K+ channel toxin from Stichodactyla helianthusas potent and selective blockers of the voltage-gated potassium channel Kv1.3". FEBS Journal. 282 (12): 2247–2259. doi:10.1111/febs.13294. ISSN 1742-464X. PMC 4472561. PMID 25864722.
{{cite journal}}: no-break space character in|title=at position 75 (help)CS1 maint: PMC format (link) - "SMART: ShKT domain annotation". smart.embl-heidelberg.de. Retrieved 2018-05-16.
- ^ Rangaraju, Srikant; Khoo, Keith; Feng, Zhiping; Crossley, George; Nugent, Daniel; Khaytin, Ilya; Pennington, Michael; Norton, Raymond; Chandy, K. George (2010-01). "Potassium Channel Modulation by A Toxin Domain in Matrix Metalloprotease 23". Biophysical Journal. 98 (3): 212a. doi:10.1016/j.bpj.2009.12.1145. ISSN 0006-3495.
{{cite journal}}: Check date values in:|date=(help); no-break space character in|first9=at position 3 (help) - ^ Nguyen, Hai M.; Galea, Charles A.; Schmunk, Galina; Smith, Brian J.; Edwards, Robert A.; Norton, Raymond S.; Chandy, K. George (2013-03-01). "Intracellular Trafficking of the KV1.3 Potassium Channel Is Regulated by the Prodomain of a Matrix Metalloprotease". Journal of Biological Chemistry. 288 (9): 6451–6464. doi:10.1074/jbc.M112.421495. ISSN 0021-9258. PMC 3585079. PMID 23300077.
{{cite journal}}: CS1 maint: PMC format (link) CS1 maint: unflagged free DOI (link) - ^ Galea, Charles A.; Nguyen, Hai M.; Chandy, K. George; Smith, Brian J.; Norton, Raymond S. (2014-04-01). "Domain structure and function of matrix metalloprotease 23 (MMP23): role in potassium channel trafficking". Cellular and Molecular Life Sciences. 71 (7): 1191–1210. doi:10.1007/s00018-013-1431-0. ISSN 1420-682X.
- ^ Moogk, Duane; da Silva, Ines Pires; Ma, Michelle W.; Friedman, Erica B.; de Miera, Eleazar Vega-Saenz; Darvishian, Farbod; Scanlon, Patrick; Perez-Garcia, Arianne; Pavlick, Anna C. (2014-12-10). "Melanoma expression of matrix metalloproteinase-23 is associated with blunted tumor immunity and poor responses to immunotherapy". Journal of Translational Medicine. 12: 342. doi:10.1186/s12967-014-0342-7. ISSN 1479-5876. PMC 4272770. PMID 25491880.
{{cite journal}}: CS1 maint: PMC format (link) CS1 maint: unflagged free DOI (link) - ^ Pan, T.-L.; Gröger, Hans; Schmid, Volker; Spring, J. (1998-07-01). "A toxin homology domain in an astacin-like metalloproteinase of the jellyfish Podocoryne carnea with a dual role in digestion and development". Development Genes and Evolution. 208 (5): 259–266. doi:10.1007/s004270050180. ISSN 0949-944X.
- Chandy, K George; Norton, Raymond S (2017-06). "Peptide blockers of K v 1.3 channels in T cells as therapeutics for autoimmune disease". Current Opinion in Chemical Biology. 38: 97–107. doi:10.1016/j.cbpa.2017.02.015. ISSN 1367-5931.
{{cite journal}}: Check date values in:|date=(help) - Gilquin, Bernard; Braud, Sandrine; Eriksson, Mats A. L.; Roux, Benoît; Bailey, Timothy D.; Priest, Birgit T.; Garcia, Maria L.; Ménez, André; Gasparini, Sylvaine (2005-07-22). "A variable residue in the pore of Kv1 channels is critical for the high affinity of blockers from sea anemones and scorpions". The Journal of Biological Chemistry. 280 (29): 27093–27102. doi:10.1074/jbc.M413626200. ISSN 0021-9258. PMID 15890656.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - Kalman, K.; Pennington, M. W.; Lanigan, M. D.; Nguyen, A.; Rauer, H.; Mahnir, V.; Paschetto, K.; Kem, W. R.; Grissmer, S. (1998-12-04). "ShK-Dap22, a potent Kv1.3-specific immunosuppressive polypeptide". The Journal of Biological Chemistry. 273 (49): 32697–32707. ISSN 0021-9258. PMID 9830012.
- Beeton, C; Pennington, MW; Wulff, H; Singh, S; Nugent, D; Crossley, G; Khaytin, I; Calabresi, PA; Chen, CY; Gutman, GA; Chandy, KG (2005). "Targeting effector memory T cells with a selective peptide inhibitor of Kv1.3 channels for therapy of autoimmune diseases". Molecular Pharmacology. 67 (4): 1369-1381. doi:10.1124/mol.104.008193.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ Kalman, K.; Pennington, M. W.; Lanigan, M. D.; Nguyen, A.; Rauer, H.; Mahnir, V.; Paschetto, K.; Kem, W. R.; Grissmer, S. (1998-12-04). "ShK-Dap22, a potent Kv1.3-specific immunosuppressive polypeptide". The Journal of Biological Chemistry. 273 (49): 32697–32707. ISSN 0021-9258. PMID 9830012.
- ^ Pennington, M. W.; Beeton, C.; Galea, C. A.; Smith, B. J.; Chi, V.; Monaghan, K. P.; Garcia, A.; Rangaraju, S.; Giuffrida, A. (2009-4). "Engineering a Stable and Selective Peptide Blocker of the Kv1.3 Channel in T Lymphocytes". Molecular Pharmacology. 75 (4): 762–773. doi:10.1124/mol.108.052704. ISSN 0026-895X. PMC 2684922. PMID 19122005.
{{cite journal}}: Check date values in:|date=(help)CS1 maint: PMC format (link) - Chang, Shih C.; Huq, Redwan; Chhabra, Sandeep; Beeton, Christine; Pennington, Michael W.; Smith, Brian J.; Norton, Raymond S. (2015-04-23). "N-terminally extended analogues of the K+ channel toxin from Stichodactyla helianthusas potent and selective blockers of the voltage-gated potassium channel Kv1.3". FEBS Journal. 282 (12): 2247–2259. doi:10.1111/febs.13294. ISSN 1742-464X. PMC 4472561. PMID 25864722.
{{cite journal}}: no-break space character in|title=at position 75 (help)CS1 maint: PMC format (link) - Pennington, M. W.; Mahnir, V. M.; Khaytin, I.; Zaydenberg, I.; Byrnes, M. E.; Kem, W. R. (January 1996). "An Essential Binding Surface for ShK Toxin Interaction with Rat Brain Potassium Channels†". Biochemistry. 35 (51): 16407–16411. doi:10.1021/bi962463g. ISSN 0006-2960.
- ^ Lanigan, Mark D.; Kalman, Katalin; Lefievre, Yann; Pennington, Michael W.; Chandy, K. George; Norton, Raymond S. (2002-10-08). "Mutating a critical lysine in ShK toxin alters its binding configuration in the pore-vestibule region of the voltage-gated potassium channel, Kv1.3". Biochemistry. 41 (40): 11963–11971. ISSN 0006-2960. PMID 12356296.
- ^ Chandy, K George; Norton, Raymond S (2017-06). "Peptide blockers of K v 1.3 channels in T cells as therapeutics for autoimmune disease". Current Opinion in Chemical Biology. 38: 97–107. doi:10.1016/j.cbpa.2017.02.015. ISSN 1367-5931.
{{cite journal}}: Check date values in:|date=(help) - ^ Rauer, H.; Pennington, M.; Cahalan, M.; Chandy, K. G. (1999-07-30). "Structural conservation of the pores of calcium-activated and voltage-gated potassium channels determined by a sea anemone toxin". The Journal of Biological Chemistry. 274 (31): 21885–21892. ISSN 0021-9258. PMID 10419508.
- Gilquin, Bernard; Braud, Sandrine; Eriksson, Mats A. L.; Roux, Benoît; Bailey, Timothy D.; Priest, Birgit T.; Garcia, Maria L.; Ménez, André; Gasparini, Sylvaine (2005-07-22). "A variable residue in the pore of Kv1 channels is critical for the high affinity of blockers from sea anemones and scorpions". The Journal of Biological Chemistry. 280 (29): 27093–27102. doi:10.1074/jbc.M413626200. ISSN 0021-9258. PMID 15890656.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - Stehling, Eliana G.; Sforça, Mauricio L.; Zanchin, Nilson I. T.; Oyama, Sérgio; Pignatelli, Angela; Belluzzi, Ottorino; Polverini, Eugenia; Corsini, Romina; Spisni, Alberto (2012-02-27). "Looking over Toxin–K+ Channel Interactions. Clues from the Structural and Functional Characterization of α-KTx Toxin Tc32, a Kv1.3 Channel Blocker". Biochemistry. 51 (9): 1885–1894. doi:10.1021/bi201713z. ISSN 0006-2960.
- ^ Kalman K, Pennington MW, Lanigan MD, Nguyen A, Rauer H, Mahnir V, Paschetto K, Kem WR, Grissmer S, Gutman GA, Christian EP, Cahalan MD, Norton RS, Chandy KG (December 1998). "ShK-Dap22, a potent Kv1.3-specific immunosuppressive polypeptide". J. Biol. Chem. 273 (49): 32697–707. doi:10.1074/jbc.273.49.32697. PMID 9830012.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ Rauer H, Pennington M, Cahalan M, Chandy KG (July 1999). "Structural conservation of the pores of calcium-activated and voltage-gated potassium channels determined by a sea anemone toxin". J. Biol. Chem. 274 (31): 21885–92. doi:10.1074/jbc.274.31.21885. PMID 10419508.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - Middleton RE, Sanchez M, Linde AR, Bugianesi RM, Dai G, Felix JP, Koprak SL, Staruch MJ, Bruguera M, Cox R, Ghosh A, Hwang J, Jones S, Kohler M, Slaughter RS, McManus OB, Kaczorowski GJ, Garcia ML (November 2003). "Substitution of a single residue in Stichodactyla helianthus peptide, ShK-Dap22, reveals a novel pharmacological profile". Biochemistry. 42 (46): 13698–707. doi:10.1021/bi035209e. PMID 14622016.
- ^ Beeton C, Pennington MW, Wulff H, Singh S, Nugent D, Crossley G, Khaytin I, Calabresi PA, Chen CY, Gutman GA, Chandy KG (April 2005). "Targeting effector memory T cells with a selective peptide inhibitor of Kv1.3 channels for therapy of autoimmune diseases". Mol. Pharmacol. 67 (4): 1369–81. doi:10.1124/mol.104.008193. PMC 4275123. PMID 15665253.
- ^ Yan L, Herrington J, Goldberg E, Dulski PM, Bugianesi RM, Slaughter RS, Banerjee P, Brochu RM, Priest BT, Kaczorowski GJ, Rudy B, Garcia ML (May 2005). "Stichodactyla helianthus peptide, a pharmacological tool for studying Kv3.2 channels". Mol. Pharmacol. 67 (5): 1513–21. doi:10.1124/mol.105.011064. PMID 15709110.
- Lanigan MD, Kalman K, Lefievre Y, Pennington MW, Chandy KG, Norton RS (October 2002). "Mutating a critical lysine in ShK toxin alters its binding configuration in the pore-vestibule region of the voltage-gated potassium channel, Kv1.3". Biochemistry. 41 (40): 11963–71. doi:10.1021/bi026400b. PMID 12356296.
- ^ Chandy KG, Wulff H, Beeton C, Pennington M, Gutman GA, Cahalan MD (May 2004). "K channels as targets for specific immunomodulation". Trends Pharmacol. Sci. 25 (5): 280–9. doi:10.1016/j.tips.2004.03.010. PMC 2749963. PMID 15120495.
- Norton RS, Pennington MW, Wulff H (December 2004). "Potassium channel blockade by the sea anemone toxin ShK for the treatment of multiple sclerosis and othfer autoimmune diseases". Curr. Med. Chem. 11 (23): 3041–52. doi:10.2174/0929867043363947. PMID 15578998.
- ^ Beeton C, Wulff H, Singh S, Botsko S, Crossley G, Gutman GA, Cahalan MD, Pennington M, Chandy KG (March 2003). "A novel fluorescent toxin to detect and investigate Kv1.3 channel up-regulation in chronically activated T lymphocytes". J. Biol. Chem. 278 (11): 9928–37. doi:10.1074/jbc.M212868200. PMID 12511563.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ Beeton C, Wulff H, Standifer NE, Azam P, Mullen KM, Pennington MW, Kolski-Andreaco A, Wei E, Grino A, Counts DR, Wang PH, LeeHealey CJ, S Andrews B, Sankaranarayanan A, Homerick D, Roeck WW, Tehranzadeh J, Stanhope KL, Zimin P, Havel PJ, Griffey S, Knaus HG, Nepom GT, Gutman GA, Calabresi PA, Chandy KG (November 2006). "Kv1.3 channels are a therapeutic target for T cell-mediated autoimmune diseases". Proc. Natl. Acad. Sci. U.S.A. 103 (46): 17414–9. doi:10.1073/pnas.0605136103. PMC 1859943. PMID 17088564.
- ^ Pennington MW, Beeton C, Galea CA, Smith BJ, Chi V, Monaghan KP, Garcia A, Rangaraju S, Giuffrida A, Plank D, Crossley G, Nugent D, Khaytin I, Lefievre Y, Peshenko I, Dixon C, Chauhan S, Orzel A, Inoue T, Hu X, Moore RV, Norton RS, Chandy KG (January 2009). "Engineering a stable and selective peptide blocker of the Kv1.3 channel in T lymphocytes". Mol. Pharmacol. 75 (4): 762–73. doi:10.1124/mol.108.052704. PMC 2684922. PMID 19122005.
- ^ Tarcha EJ, Chi V, Muñoz-Elias EJ, Bailey D, Londono LM, Upadhyay SK, Norton KN, Olson A, Tjong I, Nguyen HM, Hu X, Rupert GW, Boley SE, Slauter R, Sams J, Knapp B, Kentala D, Hansen Z, Pennington MW, Beeton C, Chandy KG, Iadonato SP (2012). "Durable pharmacological responses from a single dose of the peptide drug ShK-186, a specific Kv1.3 channel inhibitor". J. Pharm. Exp. Therap. 342 (3): 642–653. doi:10.1124/jpet.112.191890. PMC 3422530. PMID 22637724.
- Beeton C, Smith BJ, Sabo JK, Crossley G, Nugent D, Khaytin I, Chi V, Chandy KG, Pennington MW, Norton RS (January 2008). "The D-diastereomer of ShK toxin selectively blocks voltage-gated K channels and inhibits T lymphocyte proliferation". J. Biol. Chem. 283 (2): 988–97. doi:10.1074/jbc.M706008200. PMID 17984097.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - Pennington MW, Rashid MH, Tajhya RB, Beeton C, Kuyucak S, Norton RS (November 2012). "A C-terminally amidated analogue of ShK is a potent and selective blocker of the voltage-gated potassium channel Kv1.3". FEBS Lett. 586 (22): 3996–4001. doi:10.1016/j.febslet.2012.09.038. PMC 3496055. PMID 23063513.
- Wulff H, Calabresi PA, Allie R, Yun S, Pennington M, Beeton C, Chandy KG (June 2003). "The voltage-gated Kv1.3 K channel in effector memory T cells as new target for MS". J. Clin. Invest. 111 (11): 1703–13. doi:10.1172/JCI16921. PMC 156104. PMID 12782673.
- ^ Matheu MP, Beeton C, Garcia A, Chi V, Rangaraju S, Safrina O, Monaghan K, Uemura MI, Li D, Pal S, de la Maza LM, Monuki E, Flügel A, Pennington MW, Parker I, Chandy KG, Cahalan MD (October 2008). "Imaging of effector memory T cells during a delayed-type hypersensitivity reaction and suppression by Kv1.3 channel block". Immunity. 29 (4): 602–14. doi:10.1016/j.immuni.2008.07.015. PMC 2732399. PMID 18835197.
- ^ Beeton C, Wulff H, Barbaria J, Clot-Faybesse O, Pennington M, Bernard D, Cahalan MD, Chandy KG, Béraud E (November 2001). "Selective blockade of T lymphocyte K channels ameliorates experimental autoimmune encephalomyelitis, a model for multiple sclerosis". Proc. Natl. Acad. Sci. U.S.A. 98 (24): 13942–7. doi:10.1073/pnas.241497298. PMC 61146. PMID 11717451.
- Wulff H, Beeton C, Chandy KG (September 2003). "Potassium channels as therapeutic targets for autoimmune disorders". Curr Opin Drug Discov Dev. 6 (5): 640–7. PMID 14579513.
- Tucker K, Overton JM, Fadool DA (August 2008). "Kv1.3 gene-targeted deletion alters longevity and reduces adiposity by increasing locomotion and metabolism in melanocortin-4 receptor-null mice". Int J Obes (Lond). 32 (8): 1222–32. doi:10.1038/ijo.2008.77. PMC 2737548. PMID 18542083.
- Xu J, Koni PA, Wang P, Li G, Kaczmarek L, Wu Y, Li Y, Flavell RA, Desir GV (March 2003). "The voltage-gated potassium channel Kv1.3 regulates energy homeostasis and body weight". Hum. Mol. Genet. 12 (5): 551–9. doi:10.1093/hmg/ddg049. PMID 12588802.
- Xu J, Wang P, Li Y, Li G, Kaczmarek LK, Wu Y, Koni PA, Flavell RA, Desir GV (March 2004). "The voltage-gated potassium channel Kv1.3 regulates peripheral insulin sensitivity". Proc. Natl. Acad. Sci. U.S.A. 101 (9): 3112–7. doi:10.1073/pnas.0308450100. PMC 365752. PMID 14981264.
- Valverde P, Kawai T, Taubman MA (June 2005). "Potassium channel-blockers as therapeutic agents to interfere with bone resorption of periodontal disease". J. Dent. Res. 84 (6): 488–99. doi:10.1177/154405910508400603. PMID 15914584.