This is an old revision of this page, as edited by CheMoBot (talk | contribs) at 16:48, 21 January 2012 (Updating {{chembox}} (changes to verified fields - added verified revid - updated 'DrugBank_Ref', 'UNII_Ref', 'ChEMBL_Ref', 'ChEBI_Ref', 'KEGG_Ref') per Chem/Drugbox validation (report [[Wikipedia_talk:WikiProject_Chemicals|errors...). The present address (URL) is a permanent link to this revision, which may differ significantly from the current revision.
Revision as of 16:48, 21 January 2012 by CheMoBot (talk | contribs) (Updating {{chembox}} (changes to verified fields - added verified revid - updated 'DrugBank_Ref', 'UNII_Ref', 'ChEMBL_Ref', 'ChEBI_Ref', 'KEGG_Ref') per Chem/Drugbox validation (report [[Wikipedia_talk:WikiProject_Chemicals|errors...)(diff) ← Previous revision | Latest revision (diff) | Newer revision → (diff)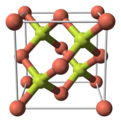 | |
| Names | |
|---|---|
| IUPAC name Copper(I) fluoride | |
| Systematic IUPAC name Fluorocopper | |
| Other names Cuprous fluoride | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| ChemSpider | |
| PubChem CID | |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | CuF |
| Molar mass | 82.544 g·mol |
| Density | 7.1 g cm |
| Structure | |
| Crystal structure | sphalerite |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |
Copper(I) fluoride is an inorganic compound with the chemical formula CuF. Its existence is uncertain. It was reported in 1933 to have a sphalerite-type crystal structure. Modern textbooks state that CuF is not known, since fluorine is so electronegative that it will always oxidise copper to its +2 oxidation state. Complexes of CuF such as are, however, known and well characterised.
Synthesis and reactivity
It can be formed by the reduction of copper(II) fluoride. Unlike copper(I) chloride, copper(I) fluoride tends to disproportionate into copper(II) fluoride and copper in a one to one ratio at ambient conditions, unless it is stabilised through complexation as in the example of .
- 2CuF → Cu + CuF2
As a result of this disproportiontion, samples slowly become light cyan in colour, the colour of copper(II) fluoride.
References
- "Copper Monofluoride - PubChem Public Chemical Database". The PubChem Project. USA: National Center for Biotechnology Information.
- Ebert, F.; Woitinek, H. (1933). "Kristallstrukturen von Fluoriden. II. HgF, HgF2, CuF und CuF2". Z. anorg. allg. Chem. 210 (3): 269–272. doi:10.1002/zaac.19332100307.
- Housecroft, C. E.; Sharpe, A. G. (2008). Inorganic Chemistry (3rd ed.). Prentice Hall. pp. 737–738. ISBN 978-0-13-175553-6.
- Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. pp. 1183–1185. ISBN 978-0-08-037941-8.
- Gulliver, D. J.; Levason, W.; Webster, M. (1981). "Coordination Stabilised Copper(I) Fluoride. Crystal and Molecular Structure of
Fluorotris(triphenylphosphine)copper(I)·Ethanol (1/2), Cu(PPh3)3F·2EtOH". Inorg. Chim. Acta. 52: 153–159. doi:10.1016/S0020-1693(00)88590-4.
{{cite journal}}: line feed character in|title=at position 80 (help)
| This article needs additional citations for verification. Please help improve this article by adding citations to reliable sources. Unsourced material may be challenged and removed. Find sources: "Copper(I) fluoride" – news · newspapers · books · scholar · JSTOR (April 2011) (Learn how and when to remove this message) |
| Copper compounds | |
|---|---|
| Cu(0,I) | |
| Cu(I) | |
| Cu(I,II) | |
| Cu(II) | |
| Cu(III) | |
| Cu(IV) | |
This inorganic compound–related article is a stub. You can help Misplaced Pages by expanding it. |