This is an old revision of this page, as edited by MartinBotIII (talk | contribs) at 16:46, 23 July 2011 (fix MSDS link (ilo.org) using AWB). The present address (URL) is a permanent link to this revision, which may differ significantly from the current revision.
Revision as of 16:46, 23 July 2011 by MartinBotIII (talk | contribs) (fix MSDS link (ilo.org) using AWB)(diff) ← Previous revision | Latest revision (diff) | Newer revision → (diff)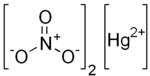 | |
| Names | |
|---|---|
| IUPAC names
Mercury dinitrate Mercury(II) nitrate | |
| Other names Mercuric nitrate | |
| Identifiers | |
| CAS Number | |
| ECHA InfoCard | 100.030.126 |
| EC Number |
|
| RTECS number |
|
| UN number | 1625 |
| CompTox Dashboard (EPA) | |
| Properties | |
| Chemical formula | Hg(NO3)2 |
| Molar mass | 324.7 g/mol |
| Appearance | colorless crystals or white powder |
| Density | 4.3 g/cm (monohydrate) |
| Melting point | 79 °C (monohydrate) |
| Solubility in water | soluble |
| Solubility | soluble in nitric acid insoluble in alcohol |
| Hazards | |
| NFPA 704 (fire diamond) |
 |
| Flash point | Non-flammable |
| Related compounds | |
| Other anions | Mercury(II) sulfate Mercury(II) chloride |
| Other cations | Zinc nitrate Cadmium nitrate |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |
Mercury(II) nitrate is a toxic colorless or white soluble crystalline mercury(II) salt of nitric acid. It was also used to treat fur to make felt in a process called 'carroting'. The phrase 'mad as a hatter' is associated with psychological illness brought on by excessive exposure to mercury(II) nitrate. The practice continued in the United States until it was banned in December 1941 by The United States Public Health Service. It is made by reacting hot concentrated nitric acid with mercury metal; dilute nitric acid would produce mercury(I) nitrate. It is an oxidizing agent.
See also
References
External links
- ATSDR - ToxFAQs: Mercury
- ATSDR - Public Health Statement: Mercury
- ATSDR - ALERT! Patterns of Metallic Mercury Exposure, 6/26/97
- ATSDR - MMG: Mercury
- ATSDR - Toxicological Profile: Mercury
- Safety data (MSDS)
- Mercuric Nitrate (ICSC)
- Mercury
- Mercury Information Packages
- How to Make Good Mercury Electrical Connections, Popular Science monthly, February 1919, Unnumbered page, Scanned by Google Books: http://books.google.com/books?id=7igDAAAAMBAJ&pg=PT14
| Mercury compounds | |||
|---|---|---|---|
| Mercury(I) | |||
| Mercury(II) |
| ||
| Mercury(IV) |
| ||
| Amalgams | |||
| Mercury cations | |||
This inorganic compound–related article is a stub. You can help Misplaced Pages by expanding it. |