| Revision as of 17:05, 31 October 2015 edit73.162.132.47 (talk) fix typo← Previous edit | Revision as of 17:28, 31 October 2015 edit undo73.162.132.47 (talk) →Available products: rearranging/movingNext edit → | ||
| Line 22: | Line 22: | ||
| The most important use of beta lactamase inhibitors is in the treatment of infections known or believed to be caused by ], as beta lactamase production is an important contributor to beta lactam resistance in these pathogens. In contrast, most beta lactam resistance in ] is due variations in ] that lead to reduced binding to the beta lactam.<ref>{{cite journal |vauthors=Georgopapadakou NH |title=Penicillin-binding proteins and bacterial resistance to beta-lactams |journal=Antimicrob. Agents Chemother. |volume=37 |issue=10 |pages=2045–53 |year=1993 |pmid=8257121 |pmc=192226 |doi= |url=}}</ref><ref>{{cite journal |vauthors=Zapun A, Contreras-Martel C, Vernet T |title=Penicillin-binding proteins and beta-lactam resistance |journal=FEMS Microbiol. Rev. |volume=32 |issue=2 |pages=361–85 |year=2008 |pmid=18248419 |doi=10.1111/j.1574-6976.2007.00095.x |url=}}</ref> The Gram-(+) pathogen ''Staphylococcus aureus'' produces beta lactamases, but beta lactamase inhibitors play a lesser role in treatment of these infections because the most resistant strains (] also use variant penicillin-binding proteins.<ref>{{cite journal |vauthors=Curello J, MacDougall C |title=Beyond Susceptible and Resistant, Part II: Treatment of Infections Due to Gram-Negative Organisms Producing Extended-Spectrum β-Lactamases |journal=J Pediatr Pharmacol Ther |volume=19 |issue=3 |pages=156–64 |year=2014 |pmid=25309145 |pmc=4187532 |doi=10.5863/1551-6776-19.3.156 |url=}}</ref><ref>{{cite journal |vauthors=Wolter DJ, Lister PD |title=Mechanisms of β-lactam resistance among Pseudomonas aeruginosa |journal=Curr. Pharm. Des. |volume=19 |issue=2 |pages=209–22 |year=2013 |pmid=22894618 |doi= |url=}}</ref> | The most important use of beta lactamase inhibitors is in the treatment of infections known or believed to be caused by ], as beta lactamase production is an important contributor to beta lactam resistance in these pathogens. In contrast, most beta lactam resistance in ] is due variations in ] that lead to reduced binding to the beta lactam.<ref>{{cite journal |vauthors=Georgopapadakou NH |title=Penicillin-binding proteins and bacterial resistance to beta-lactams |journal=Antimicrob. Agents Chemother. |volume=37 |issue=10 |pages=2045–53 |year=1993 |pmid=8257121 |pmc=192226 |doi= |url=}}</ref><ref>{{cite journal |vauthors=Zapun A, Contreras-Martel C, Vernet T |title=Penicillin-binding proteins and beta-lactam resistance |journal=FEMS Microbiol. Rev. |volume=32 |issue=2 |pages=361–85 |year=2008 |pmid=18248419 |doi=10.1111/j.1574-6976.2007.00095.x |url=}}</ref> The Gram-(+) pathogen ''Staphylococcus aureus'' produces beta lactamases, but beta lactamase inhibitors play a lesser role in treatment of these infections because the most resistant strains (] also use variant penicillin-binding proteins.<ref>{{cite journal |vauthors=Curello J, MacDougall C |title=Beyond Susceptible and Resistant, Part II: Treatment of Infections Due to Gram-Negative Organisms Producing Extended-Spectrum β-Lactamases |journal=J Pediatr Pharmacol Ther |volume=19 |issue=3 |pages=156–64 |year=2014 |pmid=25309145 |pmc=4187532 |doi=10.5863/1551-6776-19.3.156 |url=}}</ref><ref>{{cite journal |vauthors=Wolter DJ, Lister PD |title=Mechanisms of β-lactam resistance among Pseudomonas aeruginosa |journal=Curr. Pharm. Des. |volume=19 |issue=2 |pages=209–22 |year=2013 |pmid=22894618 |doi= |url=}}</ref> | ||
| == |
==Mechanism of action== | ||
| The main classes of beta lactam antibiotics used to treat Gram-(-) bacterial infections include (in approximate order of intrinsic resistance to cleavage by beta lactamases) penicillins (especially aminopenicillins and ureidopenicillins), 3rd generation cephalosporins, and carbapenems. Individual beta lactamase variants may target one or many of these drug classes, and only a subset will be inhibited by a given beta lactamase inhibitor.<ref>{{cite journal |vauthors=Drawz SM, Bonomo RA |title=Three decades of beta-lactamase inhibitors |journal=Clin. Microbiol. Rev. |volume=23 |issue=1 |pages=160–201 |year=2010 |pmid=20065329 |pmc=2806661 |doi=10.1128/CMR.00037-09 |url=}}</ref> | |||
| The main classes of beta lactam antibiotics used to treat Gram-(-) bacterial infections include penicillins (especially aminopenicillins and ureidopenicillins), 3rd generation cephalosporins, and carbapenems. Individual beta lactamase variants may target one or many of these drug classes, and only a subset will be inhibited by a given beta lactamase inhibitor. Novel transition state mimicking inhibitor with strong inhibitory potential towards beta-lactamases of deadly pathogens brings new hope to antibacterial therapeutics.<ref>{{cite journal |last1=Hazra |first1=Saugata |last2=Kurz |first2=Sebastian G. |last3=Bethel |first3=Christopher |last4=Romagnoli |first4=Chiara |last5=Caselli |first5=Emilia |last6=Prati |first6=Fabio |last7=Blanchard |first7=John S. |last8=Bonomo |first8=Robert A. |year=2015 |title=Inhibiting the β-Lactamase of Mycobacterium tuberculosis (Mtb) with Novel Boronic-Acid-Transition-State-Inhibitors (BATSIs) |journal=ACS Infectious Diseases |doi=10.1021/acsinfecdis.5b00003}}</ref> | |||
| ==Commonly used agents== | ==Commonly used agents== | ||
Revision as of 17:28, 31 October 2015
 Clavulanic acid
Clavulanic acid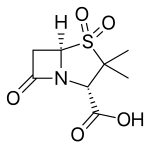 Sulbactam
Sulbactam Tazobactam
Tazobactam Avibactam
Avibactam
Beta lactamases are a family of enzymes involved in bacterial resistance to beta-lactam antibiotics. They act by breaking the beta-lactam ring that allows penicillin-like antibiotics to work. Strategies for combatting this form of resistance have included the development of new beta lactam antibiotics that are more resistant to cleavage, and the development of beta lactamase inhibitors. Although β-lactamase inhibitors have little antibiotic activity of their own, they prevent bacterial degradation of beta lactam antibiotics and thus extend the range of bacteria the drugs are effective against.
Medical uses
The most important use of beta lactamase inhibitors is in the treatment of infections known or believed to be caused by Gram-(-) bacteria, as beta lactamase production is an important contributor to beta lactam resistance in these pathogens. In contrast, most beta lactam resistance in Gram-(+) bacteria is due variations in penicillin-binding proteins that lead to reduced binding to the beta lactam. The Gram-(+) pathogen Staphylococcus aureus produces beta lactamases, but beta lactamase inhibitors play a lesser role in treatment of these infections because the most resistant strains (methicillin-resistant Staphylococcus aureus also use variant penicillin-binding proteins.
Mechanism of action
The main classes of beta lactam antibiotics used to treat Gram-(-) bacterial infections include (in approximate order of intrinsic resistance to cleavage by beta lactamases) penicillins (especially aminopenicillins and ureidopenicillins), 3rd generation cephalosporins, and carbapenems. Individual beta lactamase variants may target one or many of these drug classes, and only a subset will be inhibited by a given beta lactamase inhibitor.
Commonly used agents
- Clavulanic acid or clavulanate, usually combined with amoxicillin (Augmentin) or ticarcillin (Timentin)
- Sulbactam, usually combined with ampicillin (Unasyn) or Cefoperazone (Sulperazon)
- Tazobactam, usually combined with piperacillin (Zosyn) (Tazocin)
- Avibactam, approved in combination with ceftazidime (Avycaz), currently undergoing clinical trials for combination with ceftaroline
Beta-lactamase producing bacteria
Bacteria that can produce beta-lactamases include, but are not limited to:
- MRSA
- Staphylococcus
- Enterobacteriaceae
- Haemophilus influenzae
- Neisseria gonorrhoeae
- Klebsiella pneumoniae
- Citrobacter
- Morganella
Research
Some bacteria can produce extended spectrum β-lactamases (ESBLs) making the infection more difficult to treat and conferring additional resistance to penicillins, cephalosporins, and carbapenems. Boronic acid derivatives are currently under vast and extensive research as novel active site inhibitors for beta-lactamases because they contain a site that mimics the transition state that beta-lactams go through when undergoing hydrolysis via beta-lactamases. They have been found generally to fit well into the active site of many beta-lactamases and have the convinient property of being unable to be hydrolysed, and therefore rendered useless. This is a favorable drug design over many clinically used competing agents, because most of them, such as clavulonic acid, become hydrolysed, and are therefore only useful for a finite period of time. This generally causes the need for a higher concentration of competitive inhibitor than would be necessary in an unhydrolyzable inhibitor. Different boronic acid derivatives have to potential to be tailored to the many different isoforms of beta-lactamses, and therefore have the potential to reestablish potency of beta-lactam antibiotics.
References
- Essack SY (2001). "The development of beta-lactam antibiotics in response to the evolution of beta-lactamases". Pharm. Res. 18 (10): 1391–9. PMID 11697463.
- "Beta-Lactamase Inhibitors". Department of Nursing of the Fort Hays State University College of Health and Life Sciences. October 2000. Archived from the original on 2007-09-27. Retrieved 2007-08-17.
- Georgopapadakou NH (1993). "Penicillin-binding proteins and bacterial resistance to beta-lactams". Antimicrob. Agents Chemother. 37 (10): 2045–53. PMC 192226. PMID 8257121.
- Zapun A, Contreras-Martel C, Vernet T (2008). "Penicillin-binding proteins and beta-lactam resistance". FEMS Microbiol. Rev. 32 (2): 361–85. doi:10.1111/j.1574-6976.2007.00095.x. PMID 18248419.
- Curello J, MacDougall C (2014). "Beyond Susceptible and Resistant, Part II: Treatment of Infections Due to Gram-Negative Organisms Producing Extended-Spectrum β-Lactamases". J Pediatr Pharmacol Ther. 19 (3): 156–64. doi:10.5863/1551-6776-19.3.156. PMC 4187532. PMID 25309145.
- Wolter DJ, Lister PD (2013). "Mechanisms of β-lactam resistance among Pseudomonas aeruginosa". Curr. Pharm. Des. 19 (2): 209–22. PMID 22894618.
- Drawz SM, Bonomo RA (2010). "Three decades of beta-lactamase inhibitors". Clin. Microbiol. Rev. 23 (1): 160–201. doi:10.1128/CMR.00037-09. PMC 2806661. PMID 20065329.
- Livermore, David M. (October 1995). "β-Lactamases in Laboratory and Clinical Resistance". Clinical Microbiology Reviews. 8 (4): 557–84. PMC 172876. PMID 8665470.
- Leonard, David A.; Bonomo, Robert A.; Powers, Rachel (2012). "Class D β‑Lactamases: A Reappraisal after
Five Decades". Accounts of Chemical Reseach.
{{cite journal}}: line feed character in|title=at position 42 (help)
External links
- Xu, Hua; Hazra, Saugata; Blanchard, John S. (2012). "NXL104 Irreversibly Inhibits the β-Lactamase from Mycobacterium tuberculosis". Biochemistry. 51 (22): 4551–7. doi:10.1021/bi300508r. PMID 22587688.
- Kurz, Sebastian G.; Wolff, Kerstin A.; Hazra, Saugata; Bethel, Christopher R.; Hujer, Andrea M.; Smith, Kerri M.; Xu, Yan; Tremblay, Lee W.; Blanchard, John S.; Nguyen, Liem; Bonomo, Robert A. (2013). "Can Inhibitor-Resistant Substitutions in the Mycobacterium tuberculosis β-Lactamase BlaC Lead to Clavulanate Resistance?: a Biochemical Rationale for the Use of β-Lactam–β-Lactamase Inhibitor Combinations". Antimicrobial Agents and Chemotherapy. 57 (12): 6085–96. doi:10.1128/AAC.01253-13. PMID 24060876.
| Pharmacology: enzyme inhibition | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Class | |||||||||||
| Substrate |
| ||||||||||