| Revision as of 19:16, 6 August 2011 editCheMoBot (talk | contribs)Bots141,565 edits Updating {{chembox}} (changes to verified fields - updated 'DrugBank_Ref', 'UNII_Ref', 'ChEMBL_Ref', 'ChEBI_Ref', 'ChEBI_Ref') per Chem/Drugbox validation (report [[Wikipedia_talk:WikiProject_Che← Previous edit |
Revision as of 02:22, 9 August 2011 edit undoCheMoBot (talk | contribs)Bots141,565 edits Updating {{chembox}} (no changed fields - added verified revid - updated 'DrugBank_Ref', 'ChEMBL_Ref', 'ChEBI_Ref', 'ChEBI_Ref') per Chem/Drugbox validation (report [[Wikipedia_talk:WikiProject_CNext edit → |
| Line 1: |
Line 1: |
|
{{chembox |
|
{{chembox |
|
⚫ |
| verifiedrevid = 443390192 |
|
| Verifiedfields = changed |
|
| ⚫ |
| verifiedrevid = 396290733 |
|
|
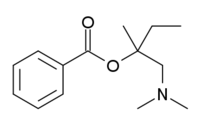
|ImageFile=Amylocaine.png |
|
|ImageFile=Amylocaine.png |
|
|ImageSize=200px |
|
|ImageSize=200px |
| Line 9: |
Line 8: |
|
| ChemSpiderID_Ref = {{chemspidercite|correct|chemspider}} |
|
| ChemSpiderID_Ref = {{chemspidercite|correct|chemspider}} |
|
| ChemSpiderID = 10312 |
|
| ChemSpiderID = 10312 |
|
| KEGG_Ref = {{keggcite|changed|kegg}} |
|
| KEGG_Ref = {{keggcite|correct|kegg}} |
|
| KEGG = D07454 |
|
| KEGG = D07454 |
|
| InChI = 1/C14H21NO2/c1-5-14(2,11-15(3)4)17-13(16)12-9-7-6-8-10-12/h6-10H,5,11H2,1-4H3 |
|
| InChI = 1/C14H21NO2/c1-5-14(2,11-15(3)4)17-13(16)12-9-7-6-8-10-12/h6-10H,5,11H2,1-4H3 |
| Line 19: |
Line 18: |
|
| CASNo=644-26-8 |
|
| CASNo=644-26-8 |
|
| PubChem=10767 |
|
| PubChem=10767 |
|
| UNII_Ref = {{fdacite|changed|FDA}} |
|
| UNII_Ref = {{fdacite|correct|FDA}} |
|
| UNII = QRW683O56T |
|
| UNII = QRW683O56T |
|
| SMILES = O=C(OC(C)(CC)CN(C)C)c1ccccc1 |
|
| SMILES = O=C(OC(C)(CC)CN(C)C)c1ccccc1 |