| Revision as of 11:40, 24 October 2011 editBeetstra (talk | contribs)Edit filter managers, Administrators172,054 edits Script assisted update of identifiers for the Chem/Drugbox validation project (updated: 'ChEMBL').← Previous edit | Latest revision as of 20:47, 8 January 2025 edit undoGraeme Bartlett (talk | contribs)Administrators250,106 edits einecs | ||
| (179 intermediate revisions by more than 100 users not shown) | |||
| Line 1: | Line 1: | ||
| {{Short description|Yellow chemical compound: building block of many dyes}} | |||
| {{chembox | {{chembox | ||
| | Verifiedfields = changed | | Verifiedfields = changed | ||
| | Watchedfields = changed | | Watchedfields = changed | ||
| | verifiedrevid = |
| verifiedrevid = 457132283 | ||
| | |
| Name = 9,10-Anthraquinone<ref name=crc/> | ||
| | |
| ImageFile = Anthraquinone acsv.svg | ||
| | ImageFile1 = Anthraquinone molecule ball from xtal.png | |||
| | ImageSize = 190px | |||
| | ImageFile2= Anthraquinone powder.jpg | |||
| | ImageName = Skeletal formula | |||
| | ImageSize2= 230px | |||
| | ImageFile1 = Anthraquinone-3D-balls.png | |||
| | PIN = Anthracene-9,10-dione<ref>{{cite book |author=] |date=2014 |title=Nomenclature of Organic Chemistry: IUPAC Recommendations and Preferred Names 2013 |publisher=] |pages=724 |doi=10.1039/9781849733069 |isbn=978-0-85404-182-4}}</ref> | |||
| | ImageSize1 = 210px | |||
| | OtherNames = {{unbulletedlist | Anthraquinone | 9,10-Anthracenedione | Anthradione | 9,10-Anthrachinon | Anthracene-9,10-quinone | 9,10-Dihydro-9,10-dioxoanthracene | Hoelite | Morkit | Corbit }} | |||
| | ImageName1 = Ball-and-stick model | |||
| | IUPACName = Anthraquinone | |||
| | OtherNames = 9,10-anthracenedione, anthradione, 9,10-anthrachinon, anthracene-9,10-quinone, 9,10-dihydro-9,10-dioxoanthracene, Hoelite, Morkit, Corbit | |||
| | Section1 = {{Chembox Identifiers | | Section1 = {{Chembox Identifiers | ||
| | SMILES = O=C1c2ccccc2C(=O)c3ccccc13 | | SMILES = O=C1c2ccccc2C(=O)c3ccccc13 | ||
| | ChemSpiderID_Ref = {{chemspidercite|correct|chemspider}} | | ChemSpiderID_Ref = {{chemspidercite|correct|chemspider}} | ||
| | ChemSpiderID = 6522 | | ChemSpiderID = 6522 | ||
| | |
| ChEBI_Ref = {{ebicite|changed|EBI}} | ||
| | ChEBI = 40448 | |||
| | ChEMBL = <!-- blanked - oldvalue: 55659 --> | |||
| | |
| ChEMBL_Ref = {{ebicite|changed|EBI}} | ||
| | |
| ChEMBL = 55659 | ||
| | |
| EC_number = 201-549-0 | ||
| | |
| CASNo_Ref = {{cascite|correct|CAS}} | ||
| | |
| CASNo = 84-65-1 | ||
| | KEGG_Ref = {{keggcite|correct|kegg}} | |||
| | KEGG = C16207 | |||
| | Gmelin = 102870 | |||
| | Beilstein = 390030 | |||
| | PubChem = 6780 | |||
| | StdInChI=1S/C14H8O2/c15-13-9-5-1-2-6-10(9)14(16)12-8-4-3-7-11(12)13/h1-8H | |||
| | StdInChIKey = RZVHIXYEVGDQDX-UHFFFAOYSA-N | |||
| | RTECS = CB4725000 | |||
| | UNNumber = 3143 | |||
| | UNII = 030MS0JBDO | |||
| }} | }} | ||
| | Section2 = {{Chembox Properties | | Section2 = {{Chembox Properties | ||
| |C=14|H=8|O=2 | | C=14 | H=8 | O=2 | ||
| | Appearance = |
| Appearance = Yellow solid | ||
| | Density = 1. |
| Density = 1.438{{nbsp}}g/cm<sup>3</sup><ref name=crc/> | ||
| | Solubility = Insoluble | | Solubility = Insoluble | ||
| | |
| MeltingPtC = 284.8 | ||
| | MeltingPt_ref = <ref name=crc>{{cite book |ref=Haynes| editor= Haynes, William M. | date = 2016| title = ] | edition = 97th | publisher = ] | isbn = 9781498754293|page=3.28}}</ref> | |||
| | BoilingPt = 379.8 °C | |||
| | BoilingPtC = 377 | |||
| | BoilingPt_ref = <ref name=crc/> | |||
| }} | }} | ||
| | Section7 = {{Chembox Hazards | | Section7 = {{Chembox Hazards | ||
| | MainHazards = | | MainHazards = possible carcinogen | ||
| | |
| FlashPtC = 185 | ||
| | |
| GHSPictograms = {{GHS08}} | ||
| | |
| GHSSignalWord = Danger | ||
| | HPhrases = {{H-phrases|350}} | |||
| | PPhrases = {{P-phrases|201|202|281|308+313|405|501}} | |||
| }} | }} | ||
| | Section8 = {{Chembox Related | | Section8 = {{Chembox Related | ||
| | |
| OtherCompounds = ],<br> ] | ||
| }} | }} | ||
| }} | }} | ||
| '''Anthraquinone''', also called '''anthracenedione''' or '''dioxoanthracene''' is an ] ] with formula {{chem|C|14|H|8|O|2}}. |
'''Anthraquinone''', also called '''anthracenedione''' or '''dioxoanthracene''', is an ] ] with formula {{chem|C|14|H|8|O|2}}. Several ]s exist but these terms usually refer to 9,10-anthraquinone (]: 9,10-dioxoanthracene) wherein the ] groups are located on the central ring. It is used as a digester additive to ] for papermaking. Many ] are generated by organisms or synthesised industrially for use as ], pharmaceuticals, and ]. Anthraquinone is a yellow, highly crystalline solid, poorly ] in ] but soluble in hot organic solvents. It is almost completely insoluble in ] near room temperature but 2.25 g will dissolve in 100 g of boiling ethanol. It is found in nature as the rare mineral ]. | ||
| ==Synthesis== | ==Synthesis== | ||
| There are several current industrial methods to produce 9,10-anthraquinone: | |||
| 9,10-Anthraquinone is obtained industrially by the oxidation of ], a reaction that is localized at the central ring. Chromium(VI) is the typical oxidant. It is also prepared by the ] of ] and ] in presence of AlCl<sub>3</sub>. The resulting o-benzoylbenzoic acid then undergoes cyclization, forming anthraquinone. This reaction is useful for producing substituted anthraquinones. The ] of ] and ] followed by oxidative dehydrogenation will also produce 9,10-anthraquinone. Lastly, ] has developed a process that proceeds via the acid-catalyzed dimerization of ] to give a 1,3-diphenylbutene, which then can be transformed to the anthaquinone.<ref name=Ullmann>Axel Vogel "Anthraquinone" in Ullmann's Encyclopedia of Industrial Chemistry 2002, Wiley-VCH, Weinheim. {{DOI|10.1002/14356007.a02_347}}</ref> It also arises via the ], a retro-Diels-Alder reaction. | |||
| # The oxidation of ]. ] is the typical oxidant. | |||
| # The ] of ] and ] in presence of ]. o-Benzoylbenzoic acid is an intermediate. This reaction is useful for producing substituted ]. | |||
| # The ] of ] and ] followed by oxidative dehydrogenation. | |||
| # The acid-catalyzed dimerization of ] to give a 1,3-diphenylbutene, which then can be transformed to the anthraquinone.<ref name=Ullmann>{{ Ullmann | author = Vogel, A. | title = Anthraquinone | doi = 10.1002/14356007.a02_347 }}</ref> This process was pioneered by ]. | |||
| It also arises via the Rickert–Alder reaction, a ]. | |||
| In a classic (1905) ] called the ], named after ] and ], anthraquinone ] with ] forming ].<ref>{{OrgSynth | title = Benzathrone | author = Macleod L.C., Allen C.F.H. | collvol = 2 | collvolpages = 62 | year = 1943 | prep = CV2P0062}}</ref> In this reaction, the quinone is first ] with ] metal in ] (converting one ] group into a ] group) after which the glycerol is added. | |||
| ==Reactions== | |||
| :] | |||
| Hydrogenation gives ] (anthrahydroquinone). Reduction with copper gives ].<ref>{{cite journal|doi= 10.15227/orgsyn.014.0004|title= Benzanthrone|journal= Organic Syntheses|year= 1934|volume= 14|pages= 4|first1=L. C. |last1= Macleod|first2=C. F. H.|last2=Allen}}</ref> Sulfonation with sulfuric acid gives anthroquinone-1-sulfonic acid,<ref>{{cite journal|doi= 10.15227/orgsyn.018.0072|title= Potassium Anthraquinone-α-Sulfonate|journal= Organic Syntheses|year= 1938|volume= 18|pages= 72|first1=W. J. |last1=Scott|first2=C. F. H.|last2=Allen}}</ref> which reacts with sodium chlorate to give 1-chloroanthaquinone.<ref>{{cite journal|doi= 10.15227/orgsyn.018.0015|title= α-Chloroanthraquinone|journal= Organic Syntheses|year= 1938|volume= 18|pages= 15|first1=W. J. |last1=Scott|first2=C. F. H.|last2=Allen}}</ref> | |||
| ==Applications |
==Applications== | ||
| {{see also|Anthraquinones}} | |||
| ===Dyestuff precursor=== | |||
| Synthetic dyes are often derived from 9,10-anthraquinone, such as ].<ref name=UllmannDye>Hans-Samuel Bien, Josef Stawitz, Klaus Wunderlich “Anthraquinone Dyes and Intermediates” Ullmann's Encyclopedia of Industrial Chemistry 2002 Wiley-VCH, Weinhem. {{DOI|10.1002/14356007.a02_355}}</ref> Important derivatives are 1-nitroanthraquinone, anthraquinone-1-sulfonic acid, and the dinitroanthraquinone.<ref name=Ullmann/> Natural ]s that are derivatives of anthraquinone are found, inter alia, in aloe latex, ], ], and ]), ], ]s, and some ]s. | |||
| [[Image:AnthDyes.png|thumb|center|750px|Selection of anthraquinone dyes. From the left: C.I.Acid Blue 43 | |||
| an "acid dye" for wool (also called "Acilan Saphirol SE"), C.I. Vat Violet 1, which is applied by transfer printing using sublimation, a blue colorant commonly used in gasoline, and C.I. Disperse Red 60, a so-called vat dye.]] | |||
| ===Digester additive in papermaking=== | ===Digester additive in papermaking=== | ||
| 9,10-Anthraquinone is used as a digester additive in production of ] by ]ne processes, like the ], the alkaline ] or the ] processes. The anthraquinone is a ] ]. The reaction mechanism may involve ] (SET).<ref>{{Cite thesis | first = J. C. | last = Samp | title = A comprehensive mechanism for anthraquinone mass transfer in alkaline pulping | year = 2008 | page = 30 | institution = Georgia Institute of Technology | hdl=1853/24767 }}</ref> The anthraquinone oxidizes the reducing end of polysaccharides in the pulp, i.e., ] and ], and thereby protecting it from alkaline degradation (peeling). The anthraquinone is reduced to ] which then can react with ]. The lignin is degraded and becomes more watersoluble and thereby more easy to wash away from the pulp, while the anthraquinone is regenerated. This process gives an increase in yield of pulp, typically 1–3% and a reduction in ].<ref>{{ cite book | editor = Goyal, G. C. | last1 = Sturgeoff | first = L. G. | last2 = Pitl | first2 = Y. | chapter = Low Kappa Pulping without Capital Investment | title = Anthraquinone Pulping | publisher = TAPPI Press | pages = 3–9 | orig-year = 1993 | year = 1997 | isbn = 0-89852-340-0 }}</ref> | |||
| === Hydrogen Peroxide Production === | |||
| ] (AMS) is a watersoluble anthraquinone derivative that was the first anthraquinone derivative discovered to have a catalytic effect in the alkaline pulping processes.<ref>{{cite web | url = http://smartech.gatech.edu/bitstream/1853/673/1/3370_001_071978.pdf | title= Anthraquinone/ alkali pulping. A literature review|first= |last= |author= |authorlink= |coauthors= |date=|month=7 |year=1978 |work= |publisher= |location= |page= |pages= |at= |language= |trans_title= |format= |doi= |archiveurl= |archivedate= |accessdate= |quote= |ref= |separator= |postscript= }}</ref> | |||
| 2-Alkyl-9,10-Anthroquinones are used as a catalyst in the ] for the production of hydrogen peroxide. This process is the dominant industrial method of hydrogen peroxide production.<ref>{{Cite journal |last=Campos-Martin |first=Jose M. |last2=Blanco-Brieva |first2=Gema |last3=Fierro |first3=Jose L. G. |date=2006 |title=Hydrogen Peroxide Synthesis: An Outlook beyond the Anthraquinone Process |url=https://onlinelibrary.wiley.com/doi/10.1002/anie.200503779 |journal=Angewandte Chemie International Edition |language=en |volume=45 |issue=42 |pages=6962–6984 |doi=10.1002/anie.200503779 |issn=1521-3773}}</ref> | |||
| ===Niche uses=== | |||
| ===In the production of hydrogen peroxide=== | |||
| 9,10-anthraquinone is used as a bird repellant on seeds, and as a gas generator in satellite balloons.<ref>{{Cite web |url=http://www.americanheritage.com/articles/magazine/it/2007/1/2007_1_38.shtml |title=www.americanheritage.com |access-date=2009-09-22 |archive-url=https://web.archive.org/web/20090609235239/http://www.americanheritage.com/articles/magazine/it/2007/1/2007_1_38.shtml |archive-date=2009-06-09 |url-status=dead }}</ref> It has also been mixed with lanolin and used as a wool spray to protect sheep flocks against ] attacks in New Zealand.<ref>{{cite news |last=Dudding |first=Adam |date=29 July 2012 |title=How to solve a problem like a kea |url=http://www.stuff.co.nz/science/7370200/How-to-solve-a-problem-like-the-kea |newspaper=Sunday Star Times |location=New Zealand |access-date=11 November 2014}}</ref> | |||
| A large industrial application of anthraquinones is for the production of ]. ] or a related alkyl derivatives is used, rather anthraquinone itself.<ref>Gustaaf Goor, Jürgen Glenneberg, Sylvia Jacobi "Hydrogen Peroxide" in Ullmann's Encyclopedia of Industrial Chemistry 2007, Wiley-VCH, Weinheim. {{DOI| 10.1002/14356007.a13_443.pub2}}.</ref> | |||
| :]{{clear-left}} | |||
| == |
==Other isomers== | ||
| Several other isomers of anthraquinone exist, including the 1,2-, 1,4-, and 2,6-anthraquinones. They are of minor importance compared to 9,10-anthraquinone. | |||
| Derivatives of 9,10-anthraquinone include many important drugs (collectively called '''anthracenediones'''). They include | |||
| * ]s such as ], ], and ], and some of the ] | |||
| * ]s such as ] | |||
| * ]s used in the treatment of ], such as ], ], and the ]s. | |||
| <table border=0 cellborder=0> | |||
| <tr valign=center> | |||
| <td align=center>]</td> | |||
| <td align=center>]</td> | |||
| <td align=center>]</td> | |||
| <tr/> | |||
| <tr valign=top> | |||
| <td align=center>Aloe emodin</td> | |||
| <td align=center>Mitoxantrone</td> | |||
| <td align=center>Pixantrone</td> | |||
| <tr/> | |||
| </table> | |||
| == |
==Safety== | ||
| Anthraquinone has no recorded {{LD50}}, probably because it is so insoluble in water. | |||
| 9,10-Anthraquinone is used as a bird repellant on seeds and as a gas generator in satellite balloons . | |||
| In terms of metabolism of substituted anthraquinones, the enzyme encoded by the gene ] has glucuronidase activity with many substrates including anthraquinones.<ref name="pmid1339448">{{ cite journal |author1= Ritter, J. K. |author2=Chen, F. |author3=Sheen, Y. Y. |author4=Tran, H. M. |author5=Kimura, S. |author6=Yeatman, M. T. |author7=Owens, I. S. | title = A Novel Complex Locus UGT1 Encodes Human Bilirubin, Phenol, and other UDP-Glucuronosyltransferase Isozymes with Identical Carboxyl Termini | journal = Journal of Biological Chemistry | year = 1992 | volume = 267 | issue = 5 | pages = 3257–3261 |doi=10.1016/S0021-9258(19)50724-4 | pmid = 1339448 | url = http://www.jbc.org/content/267/5/3257.full.pdf |doi-access=free }}</ref> | |||
| Natural anthraquinone derivatives tend to have ] effects. Prolonged use and ] leads to ].<ref>{{cite journal |author=Müller-Lissner SA |title=Adverse effects of laxatives: fact and fiction |journal=Pharmacology |volume=47 |issue=Suppl 1 |pages=138–45 |year=1993 |pmid=8234421 |doi=10.1159/000139853}}</ref><ref name="pmid3280173">{{cite journal |author=Moriarty KJ, Silk DB |title=Laxative abuse |journal=Dig Dis |volume=6 |issue=1 |pages=15–29 |year=1988 |pmid=3280173 |doi=10.1159/000171181 }}</ref> 5 anthraquinones have been shown to inhibit the formation of Tau aggregates and dissolve paired helical filaments thought to be critical to Alzheimer's disease progression in both mouse models and in vitro testing but have not been investigated as a therapeutic agent. <ref>.</ref> | |||
| == |
== See also == | ||
| * ] | |||
| Several other isomers of anthraquinone are possible, including the 1,2-, 1,4-, and 2,6-anthraquinones. They are of comparatively minor importance. The term is also used in the more general sense of any compound that can be viewed as an anthraquinone with some ] atoms replaced by other atoms or ]s. These derivatives include substances that are technically useful or play important roles in living beings. | |||
| * ] | |||
| * ] | |||
| ==See also== | |||
| * ] | |||
| *] | |||
| *] | |||
| *] | |||
| ==References== | ==References== | ||
| Line 102: | Line 100: | ||
| ==External links== | ==External links== | ||
| * |
* | ||
| * |
* | ||
| {{Natural phenol}} | |||
| {{Anthraquinone}} | |||
| {{Authority control}} | |||
| {{Organic reactions}} | |||
| ] | ] | ||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
Latest revision as of 20:47, 8 January 2025
Yellow chemical compound: building block of many dyes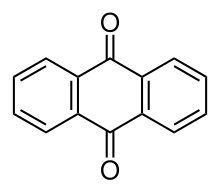 | |
 | |
 | |
| Names | |
|---|---|
| Preferred IUPAC name Anthracene-9,10-dione | |
Other names
| |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| Beilstein Reference | 390030 |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.001.408 |
| EC Number |
|
| Gmelin Reference | 102870 |
| KEGG | |
| PubChem CID | |
| RTECS number |
|
| UNII | |
| UN number | 3143 |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | C14H8O2 |
| Molar mass | 208.216 g·mol |
| Appearance | Yellow solid |
| Density | 1.438 g/cm |
| Melting point | 284.8 °C (544.6 °F; 558.0 K) |
| Boiling point | 377 °C (711 °F; 650 K) |
| Solubility in water | Insoluble |
| Hazards | |
| Occupational safety and health (OHS/OSH): | |
| Main hazards | possible carcinogen |
| GHS labelling: | |
| Pictograms | 
|
| Signal word | Danger |
| Hazard statements | H350 |
| Precautionary statements | P201, P202, P281, P308+P313, P405, P501 |
| Flash point | 185 °C (365 °F; 458 K) |
| Related compounds | |
| Related compounds | quinone, anthracene |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |
Anthraquinone, also called anthracenedione or dioxoanthracene, is an aromatic organic compound with formula C
14H
8O
2. Several isomers exist but these terms usually refer to 9,10-anthraquinone (IUPAC: 9,10-dioxoanthracene) wherein the keto groups are located on the central ring. It is used as a digester additive to wood pulp for papermaking. Many anthraquinone derivatives are generated by organisms or synthesised industrially for use as dyes, pharmaceuticals, and catalysts. Anthraquinone is a yellow, highly crystalline solid, poorly soluble in water but soluble in hot organic solvents. It is almost completely insoluble in ethanol near room temperature but 2.25 g will dissolve in 100 g of boiling ethanol. It is found in nature as the rare mineral hoelite.
Synthesis
There are several current industrial methods to produce 9,10-anthraquinone:
- The oxidation of anthracene. Chromium(VI) is the typical oxidant.
- The Friedel-Crafts reaction of benzene and phthalic anhydride in presence of AlCl3. o-Benzoylbenzoic acid is an intermediate. This reaction is useful for producing substituted anthraquinones.
- The Diels-Alder reaction of naphthoquinone and butadiene followed by oxidative dehydrogenation.
- The acid-catalyzed dimerization of styrene to give a 1,3-diphenylbutene, which then can be transformed to the anthraquinone. This process was pioneered by BASF.
It also arises via the Rickert–Alder reaction, a retro-Diels–Alder reaction.
Reactions
Hydrogenation gives dihydroanthraquinone (anthrahydroquinone). Reduction with copper gives anthrone. Sulfonation with sulfuric acid gives anthroquinone-1-sulfonic acid, which reacts with sodium chlorate to give 1-chloroanthaquinone.
Applications
See also: AnthraquinonesDigester additive in papermaking
9,10-Anthraquinone is used as a digester additive in production of paper pulp by alkaline processes, like the kraft, the alkaline sulfite or the Soda-AQ processes. The anthraquinone is a redox catalyst. The reaction mechanism may involve single electron transfer (SET). The anthraquinone oxidizes the reducing end of polysaccharides in the pulp, i.e., cellulose and hemicellulose, and thereby protecting it from alkaline degradation (peeling). The anthraquinone is reduced to 9,10-dihydroxyanthracene which then can react with lignin. The lignin is degraded and becomes more watersoluble and thereby more easy to wash away from the pulp, while the anthraquinone is regenerated. This process gives an increase in yield of pulp, typically 1–3% and a reduction in kappa number.
Hydrogen Peroxide Production
2-Alkyl-9,10-Anthroquinones are used as a catalyst in the Anthraquinone Process for the production of hydrogen peroxide. This process is the dominant industrial method of hydrogen peroxide production.
Niche uses
9,10-anthraquinone is used as a bird repellant on seeds, and as a gas generator in satellite balloons. It has also been mixed with lanolin and used as a wool spray to protect sheep flocks against kea attacks in New Zealand.
Other isomers
Several other isomers of anthraquinone exist, including the 1,2-, 1,4-, and 2,6-anthraquinones. They are of minor importance compared to 9,10-anthraquinone.
Safety
Anthraquinone has no recorded LD50, probably because it is so insoluble in water.
In terms of metabolism of substituted anthraquinones, the enzyme encoded by the gene UGT1A8 has glucuronidase activity with many substrates including anthraquinones.
See also
References
- ^ Haynes, William M., ed. (2016). CRC Handbook of Chemistry and Physics (97th ed.). CRC Press. p. 3.28. ISBN 9781498754293.
- International Union of Pure and Applied Chemistry (2014). Nomenclature of Organic Chemistry: IUPAC Recommendations and Preferred Names 2013. The Royal Society of Chemistry. p. 724. doi:10.1039/9781849733069. ISBN 978-0-85404-182-4.
- Vogel, A. "Anthraquinone". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a02_347. ISBN 978-3527306732.
- Macleod, L. C.; Allen, C. F. H. (1934). "Benzanthrone". Organic Syntheses. 14: 4. doi:10.15227/orgsyn.014.0004.
- Scott, W. J.; Allen, C. F. H. (1938). "Potassium Anthraquinone-α-Sulfonate". Organic Syntheses. 18: 72. doi:10.15227/orgsyn.018.0072.
- Scott, W. J.; Allen, C. F. H. (1938). "α-Chloroanthraquinone". Organic Syntheses. 18: 15. doi:10.15227/orgsyn.018.0015.
- Samp, J. C. (2008). A comprehensive mechanism for anthraquinone mass transfer in alkaline pulping (Thesis). Georgia Institute of Technology. p. 30. hdl:1853/24767.
- Sturgeoff, L. G.; Pitl, Y. (1997) . "Low Kappa Pulping without Capital Investment". In Goyal, G. C. (ed.). Anthraquinone Pulping. TAPPI Press. pp. 3–9. ISBN 0-89852-340-0.
- Campos-Martin, Jose M.; Blanco-Brieva, Gema; Fierro, Jose L. G. (2006). "Hydrogen Peroxide Synthesis: An Outlook beyond the Anthraquinone Process". Angewandte Chemie International Edition. 45 (42): 6962–6984. doi:10.1002/anie.200503779. ISSN 1521-3773.
- "www.americanheritage.com". Archived from the original on 2009-06-09. Retrieved 2009-09-22.
- Dudding, Adam (29 July 2012). "How to solve a problem like a kea". Sunday Star Times. New Zealand. Retrieved 11 November 2014.
- Ritter, J. K.; Chen, F.; Sheen, Y. Y.; Tran, H. M.; Kimura, S.; Yeatman, M. T.; Owens, I. S. (1992). "A Novel Complex Locus UGT1 Encodes Human Bilirubin, Phenol, and other UDP-Glucuronosyltransferase Isozymes with Identical Carboxyl Termini" (PDF). Journal of Biological Chemistry. 267 (5): 3257–3261. doi:10.1016/S0021-9258(19)50724-4. PMID 1339448.
External links
- National Pollutant Inventory — Polycyclic Aromatic Hydrocarbon Fact Sheet
- Molecules Spontaneously Form Honeycomb Network