| Revision as of 00:01, 20 April 2011 editCheMoBot (talk | contribs)Bots141,565 edits Updating {{chembox}} (no changed fields - added verified revid - updated 'UNII_Ref', 'ChemSpiderID_Ref', 'StdInChI_Ref', 'StdInChIKey_Ref', 'ChEMBL_Ref', 'KEGG_Ref') per Chem/Drugbox validation (← Previous edit | Latest revision as of 13:59, 14 May 2024 edit undoInnerstream (talk | contribs)Autopatrolled, Extended confirmed users4,131 editsNo edit summary | ||
| (20 intermediate revisions by 16 users not shown) | |||
| Line 1: | Line 1: | ||
| {{Orphan|date=February 2009}} | |||
| {{chembox | {{chembox | ||
| |Verifiedfields = changed | |||
| ⚫ | | |
||
| |Watchedfields = changed | |||
| ⚫ | | |
||
| ⚫ | |verifiedrevid = 424942077 | ||
| ⚫ | | |
||
| ⚫ | |Name = Bis-HPPP | ||
| ⚫ | | |
||
| ⚫ | |ImageFile = Bis-HPPP.svg | ||
| | ImageName = | |||
| ⚫ | |ImageSize = 200px | ||
| | IUPACName = 2,2-bispropane | |||
| |PIN = 3,3′-di(propane-1,2-diol) | |||
| ⚫ | | |
||
| |OtherNames = BADGE·2H<sub>2</sub>O | |||
| ⚫ | | |
||
| ⚫ | |Section1 = {{Chembox Identifiers | ||
| ⚫ | | |
||
| ⚫ | |CASNo = 5581-32-8 | ||
| ⚫ | |||
| |UNII_Ref = {{fdacite|correct|FDA}} | |||
| ⚫ | | |
||
| |UNII = S5K2510L3D | |||
| | Formula = C<sub>21</sub>H<sub>28</sub>O<sub>6</sub> | |||
| ⚫ | |SMILES = OCC(O)COc1ccc(cc1)C(C)(C)c2ccc(OCC(O)CO)cc2 | ||
| | MolarMass = 376.44 g/mol | |||
| |PubChem = 110678 | |||
| | Density = | |||
| |ChemSpiderID_Ref = {{chemspidercite|changed|chemspider}} | |||
| | MeltingPt = | |||
| |ChemSpiderID = 99341 | |||
| | BoilingPt = | |||
| |InChI = 1/C21H28O6/c1-21(2,15-3-7-19(8-4-15)26-13-17(24)11-22)16-5-9-20(10-6-16)27-14-18(25)12-23/h3-10,17-18,22-25H,11-14H2,1-2H3 | |||
| ⚫ | |||
| |InChIKey = NISVZEWKUNUGQQ-UHFFFAOYAT | |||
| |StdInChI_Ref = {{stdinchicite|changed|chemspider}} | |||
| |StdInChI = 1S/C21H28O6/c1-21(2,15-3-7-19(8-4-15)26-13-17(24)11-22)16-5-9-20(10-6-16)27-14-18(25)12-23/h3-10,17-18,22-25H,11-14H2,1-2H3 | |||
| |StdInChIKey_Ref = {{stdinchicite|changed|chemspider}} | |||
| |StdInChIKey = NISVZEWKUNUGQQ-UHFFFAOYSA-N | |||
| ⚫ | }} | ||
| ⚫ | |Section2 = {{Chembox Properties | ||
| |C = 21 | H = 28 | O = 6 | |||
| ⚫ | }} | ||
| }} | }} | ||
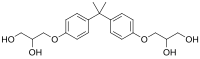
| '''Bis-HPPP''' ('''2,2-bispropane''') is an ] that is formed when the ] material ] is degraded by ] ]s.<ref>{{cite journal|last1=Shokati|first1=Babak|last2=Tam|first2=Laura Eva|last3=Santerre|first3=J. Paul|last4=Finer|first4=Yoav|title=Effect of salivary esterase on the integrity and fracture toughness of the dentin-resin interface|journal=Journal of Biomedical Materials Research Part B: Applied Biomaterials|date=2010|volume=94|issue=1|pages=230–7|doi=10.1002/jbm.b.31645|pmid=20524199}}</ref> It is also called '''BADGE·2H<sub>2</sub>O''' in reference to it being the hydrolyzed form of ], which is used in the formation of ].<ref>{{cite journal |last1=Yonekubo |first1=Jun |last2=Hayakawa |first2=Kazuichi |last3=Sajiki |first3=Junko |title=Concentrations of Bisphenol A, Bisphenol A Diglycidyl Ether, and Their Derivatives in Canned Foods in Japanese Markets |journal=Journal of Agricultural and Food Chemistry |date=1 March 2008 |volume=56 |issue=6 |pages=2041–2047 |doi=10.1021/jf073106n}}</ref> Structurally, it is a di-] of ]. | |||
| '''2,2-Bispropane''' ('''bis-HPPP''') is an organic compound structurally related to ]. | |||
| == Formation == | |||
| Together with ], bis-HPPP is released following the CE-catalyzed hydrolysis of 2,2-propane (bis-GMA). This reaction is very common in hydrolytic degradation of the dental resin since salivary esterases are able to cleave the ester bonds in ] polymers of dental composites. | Together with ], bis-HPPP is released following the CE-catalyzed{{huh|date=April 2022}} hydrolysis of 2,2-propane (bis-GMA). This reaction is very common in hydrolytic degradation of the dental resin since salivary esterases are able to cleave the ester bonds in ] polymers of dental composites. | ||
| Analysis by ] demonstrated that ] reactions would cleave the ] bonds of both methacrylate units in bis-GMA and produce bis-HPPP along with two molecules of methacrylic acid.<ref>{{cite journal | author = Finer Y, S.J. | title = The influence of resin chemistry on a dental composite's biodegradation | journal = ] | year = 2004 | volume = 69A | pages = 233–246 | doi = 10.1002/jbm.a.30000 | pmid = 15057996 | last2 = Santerre | first2 = JP | issue = 2}}</ref> | Analysis by ] demonstrated that ] reactions would cleave the ] bonds of both methacrylate units in bis-GMA and produce bis-HPPP along with two molecules of methacrylic acid.<ref>{{cite journal | author = Finer Y, S.J. | title = The influence of resin chemistry on a dental composite's biodegradation | journal = ] | year = 2004 | volume = 69A | pages = 233–246 | doi = 10.1002/jbm.a.30000 | pmid = 15057996 | last2 = Santerre | first2 = JP | issue = 2| doi-access = free }}</ref> | ||
| ==References== | ==References== | ||
| <references/> | <references/> | ||
| ] | |||
| ] | ] | ||
| ] | ] | ||
| ] | |||
Latest revision as of 13:59, 14 May 2024
 | |
| Names | |
|---|---|
| Preferred IUPAC name 3,3′-di(propane-1,2-diol) | |
| Other names BADGE·2H2O | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| ChemSpider | |
| ECHA InfoCard | 100.024.524 |
| PubChem CID | |
| UNII | |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | C21H28O6 |
| Molar mass | 376.449 g·mol |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |
Bis-HPPP (2,2-bispropane) is an organic compound that is formed when the dental composite material bis-GMA is degraded by salivary esterases. It is also called BADGE·2H2O in reference to it being the hydrolyzed form of BADGE, which is used in the formation of epoxy resins. Structurally, it is a di-ether of bisphenol A.
Formation
Together with methacrylic acid, bis-HPPP is released following the CE-catalyzed hydrolysis of 2,2-propane (bis-GMA). This reaction is very common in hydrolytic degradation of the dental resin since salivary esterases are able to cleave the ester bonds in acrylic polymers of dental composites.
Analysis by mass spectrometry demonstrated that hydrolytic reactions would cleave the ester bonds of both methacrylate units in bis-GMA and produce bis-HPPP along with two molecules of methacrylic acid.
References
- Shokati, Babak; Tam, Laura Eva; Santerre, J. Paul; Finer, Yoav (2010). "Effect of salivary esterase on the integrity and fracture toughness of the dentin-resin interface". Journal of Biomedical Materials Research Part B: Applied Biomaterials. 94 (1): 230–7. doi:10.1002/jbm.b.31645. PMID 20524199.
- Yonekubo, Jun; Hayakawa, Kazuichi; Sajiki, Junko (1 March 2008). "Concentrations of Bisphenol A, Bisphenol A Diglycidyl Ether, and Their Derivatives in Canned Foods in Japanese Markets". Journal of Agricultural and Food Chemistry. 56 (6): 2041–2047. doi:10.1021/jf073106n.
- Finer Y, S.J.; Santerre, JP (2004). "The influence of resin chemistry on a dental composite's biodegradation". J Biomed Mater Res. 69A (2): 233–246. doi:10.1002/jbm.a.30000. PMID 15057996.